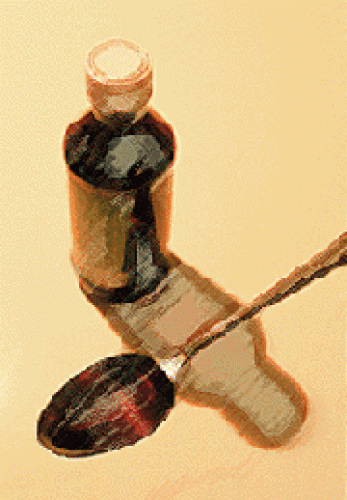Every day, we come in contact with countless microorganisms without knowing it. Even if those “bugs” set up shop in our throats or guts, we don’t get sick. But the same bugs can be devastating for people whose immune systems are impaired.
Rick Tidwell, professor of pathology, wants to change that. Five years ago, Tidwell put together a consortium, funded by the National Institutes of Health (NIH), aimed at creating new drugs to treat “opportunistic infections”-the ones that plague people whose immune systems are injured by disease, chemotherapy, or immunosuppressant drugs.
Now, the consortium is finishing pre-clinical trials of a drug to treat cryptosporidosis, a form of diarrhea that has no cure. The disease is caused by Cryptosporidium parvum, a protozoan which is found in the water supply and cannot be removed with the usual chemicals and filters. In most people, the disease occurs rarely and clears up on its own, but it can cause a devastating wasting syndrome in people with AIDS and other immune-suppressing conditions. The only treatment available now is to replenish lost fluids and hope for the best.
“We put a high priority on this drug because there are no options for people with this disease,” Tidwell says. “And the goal of these NIH consortia is to turn ideas into products as quickly as possible.”
A second drug, intended to treat pneumocystic pneumonia, which plagues people with AIDS, should be ready to enter clinical trials within the next year, Tidwell says. Both drugs are loosely based on pentamidine, one of the current treatments for pneumocystic pneumonia. Although pentamidine is effective, it can have severe side effects, including damage to the heart, liver, and kidneys. For a long time, nobody knew how to prevent those side effects because nobody knew how pentamidine worked.
Tidwell and his lab began working on that problem back in 1986. They knew that pentamidine was a dication (dye-cat-eye-on)—a molecule with two positively charged elements. But that didn’t tell them how the drug killed microorganisms, so they began to look at its effects on the protozoan Giardia. As most backpackers know, Giardia can be found in fresh-water streams and lakes and causes “beaver fever,” or diarrhea.
Tidwell’s group learned that dications bind to Giardia’s DNA. The binding itself does not kill the microorganisms, but it seems to interfere with enzymes such as topoisomerase II, which is necessary for replicating DNA when cells divide. Although dications also bind to DNA in mammals, topoisomerase II can still do its job. Pentamidine’s bad side effects seem to be caused by the disruption of different enzymes.
With that information, it became possible to design new compounds that had fewer side effects but were more effective against microorganisms. That’s when Tidwell assembled the consortium of researchers, including James Edwin Hall, associate professor of epidemiology, Dave Boykin and Dave Wilson at Georgia State University, Christine Dykstra and Byron Blagburn at Auburn University, and John Perfect at Duke University. He also invited PharmEco, a company in Lexington, Massachusetts, that specializes in drug synthesis and Immtech, a pharmaceutical company in Evanston, Illinois. Under the terms of a license from UNC-CH, the companies will commercialize new drug treatments resulting from inventions made by the consortium.
In 1992 the consortium received an NIH National Cooperative Drug Development Group grant and began to study anti-microbial compounds, including the two that are nearly ready for clinical trials.
“The cryptosporidiosis drug works because it fights the organism on its own ground—in the digestive system of an animal or person,” Tidwell says. “A person takes a pill, and the drug is delivered right where it needs to go. But the drug isn’t absorbed by the person’s body because it is a highly charged molecule. It just doesn’t cross the membrane lining the intestines. So it never gets into the bloodstream and can’t cause side effects.”
Although the dication’s charge is ideal for treating cryptosporidiosis, treating pneumocystis pneumonia is another matter. The drug needs to get into the bloodstream in order to reach the lungs. Initially, Tidwell says, the drug had to be given intravenously.
Then Tidwell and Hall came up with a solution: “cap” the molecule to reduce its charge. The body will be able to absorb the drug, and, once it is in the bloodstream, enzymes will remove the cap to expose the active form of the drug. Around the same time, a researcher in Germany, Bernd Clement, came up with a similar solution.
The capped, or “pro-drug,” form has been made into a liquid, similar to cough syrup. Because the drug no longer has to be given intravenously, the group can explore treatments for a wider range of diseases. In particular, the researchers are working with Walter Reed Army Hospital to develop an anti-malarial drug.
We had known for a long time that our compounds were very effective against malaria,” says Tidwell. “But an intravenous drug could do more harm than good in a country where money is so tight that people reuse needles. Now that we have this method of making pro-drugs, it has really reignited interest in using these compounds as anti-malarial agents.”
The consortium also has begun exploring treatments for other diseases. John Perfect’s lab at Duke University is helping to develop drugs that kill fungi. Tidwell says some compounds have already been tested against the fungi Aspergillus, Cryptococcus, and Candida, which can cause topical infections, such as thrush, an infection of the mouth, or more serious problems in the lungs and other organs. And, with help from the Southern Research Institute in Alabama, the consortium has found compounds that work against the bacterium that causes tuberculosis, Mycobacterium tuberculosis, and the closely related Mycobacterium avum.
“It’s critical to have new drugs for tuberculosis,” Tidwell says, “because many of the infections occurring now are caused by bacteria that are resistant to antibiotics.”
Tidwell says the compounds don’t seem to work the same way against bacteria as they do against protozoans. The drugs still bind to bacterial DNA, but bacteria don’t have topoisomerase II. Although bacteria do have an analogous enzyme, gyrase, it doesn’t seem to be affected by dications.
However, the researchers do know that some of their compounds are more powerful than Rifampin, the strongest tuberculosis treatment now available. That’s what’s important to Tidwell: the new drugs are meeting a need that antibiotics can’t.
“The real strength of the consortium has been that we’ve been able to develop drugs that people need quickly,” Tidwell says. “That’s really the goal. It’s nice for scientists to come up with great ideas in the laboratory, but what is really important is for the public, who paid for all the research, to get some benefit.”
Tidwell is in the Department of Pathology and Laboratory Medicine in the School of Medicine. Hall is in the Department of Epidemiology in the School of Public Health.
The Office of Technology Development (OTD) manages invention licensing and helps form start-up companies based on UNC-CH inventions. For more information, contact OTD at 919/966-3929 or Campus Box 4105, 308 Bynum Hall, UNC-CH, Chapel Hill, NC 27599-4105.