It’s Friday after work and I’m walking with Bob Goldstein to his car. I’ve known Bob for a few years, so when we get to the parking deck I look for his Saturn. He points me instead to a new Honda, and we get in. Not too long ago, he explains, he was driving the Saturn back from a grant-review panel meeting in D.C. when the oil light came on. It went right back off again, so he drove on home. That was the old Saturn’s next-to-last drive. The last was to the junkyard.
Hence the Honda, in which Bob is now giving me a lift home. He’d invited me to his lab to tell me about a new paper he has coming out in Science. (I say “he” has a paper coming out, but Bob is the first to point out that his graduate students and postdocs, current and former, made the discoveries.) The paper has to do with how cells move from the surface of an embryo to its interior.
Every complex organism, from the great white shark to your third-grade teacher, starts as a single fertilized egg cell. In vastly oversimplified terms, if that one fertilized cell divides enough times, it will become an embryo. And if all goes well, that embryo will eventually become a fully developed organism. (The tiny roundworm C. elegans, one of the organisms Bob’s lab studies, pulls this trick off in fourteen hours. That is, it starts as a fertilized egg cell and crawls away fourteen hours later with a fully functioning mouth, nervous system, muscles, and everything else it needs to live out its wormy little life.)
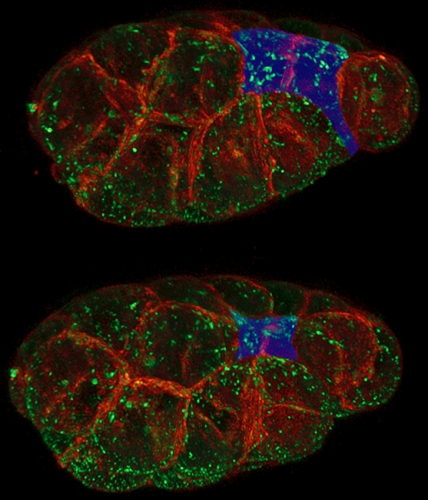
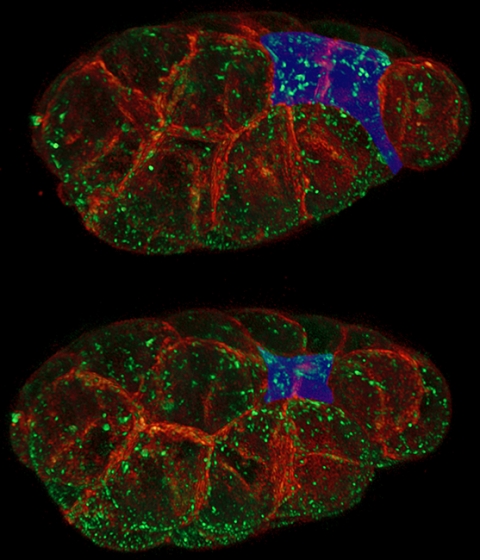
As an embryo develops, some of its cells begin to move from the surface of the embryo to its interior, where they will begin to form different tissues—and eventually different structures—in the organism: the gut, the spinal cord, and so on. It’s a critical process: Bob says our own brains and spinal cords develop from a sheet of surface cells that folds inward and eventually forms what’s called the neural tube. Neural-tube birth defects such as spina bifida are examples of the failure of that tube to properly close.
To move these surface cells to the interior, Bob says, an embryo pinches its outer surface down, effectively shrinking it. Scientists have known that this shrinking happens when a protein motor, called myosin, contracts a mesh of filaments, called actin, which is just under the cell’s surface. Conventional wisdom was that starting up this myosin motor would pull on the actin and shrink the cell surface.
Not exactly, discovered Bob’s grad student Minna Roh-Johnson. She found that the myosin motor runs for quite some time before the cell surface really begins to shrink. “This suggests that just as in a car, turning on the motor isn’t enough,” Bob says. The shrinking must be triggered by a clutch that somehow links the myosin motors to the cell surface. That discovery may lead researchers working in this area to focus less on how myosin motors get started and more on identifying and understanding the clutch.
The idea of cellular clutches isn’t new—they’re known to operate in the tips of growing nerve cells and in the long tails that some bacteria use for movement. But Bob and his lab are the first ones to propose that embryos might be using a clutch to move cells around to form animals.
Bob is doing his best to explain all of this to me as he drives me home. As he pulls smoothly away from a light, I notice the new Honda has a stick shift. Did the old Saturn have one, too? “Yeah,” he says. “But I understood clutches in cells before I understood the clutch in my car.”
Bob Goldstein is a professor of biology in the College of Arts and Sciences. Study coauthors include Minna Roh-Johnson, formerly a graduate student in Goldstein’s lab; Gidi Shemer, a former postdoc in Goldstein’s lab; and Chris Higgins, a current Goldstein-lab graduate student. The National Institutes of Health funded their research.