When her phone rang in 2004, Carol Otey had no idea it would change the course of her research.
The call was from a stranger, Teri Brentnall at the University of Washington. Brentnall had done a genetic analysis of a Washington State family, referred to by researchers as Family X, that had an extremely high rate of pancreatic cancer.
Seven members of Family X had already chosen to have their pancreases removed—giving them instant diabetes—to prevent this deadly cancer. Brentnall knew generally where the mutation was that was causing their disease, but that left about 250 candidate genes. Number twenty on the list was palladin.
Otey had discovered palladin years before. Now a stranger from across the country was calling to ask whether she thought it was possible that a mutation in palladin could be causing pancreatic cancer in Family X. Otey hesitated. A cold call like this is rare in science, where competition for funding and publications discourages tipping one’s hand.
Brentnall didn’t know that Otey had brand-new evidence that palladin affects the behavior of cultured breast cancer cells. “I had to make a decision on the spot about whether to confide in someone I had never met about our unpublished results,” Otey says. “But I felt it was necessary that I tell her.” She passed along what they knew and offered to help. Then she didn’t hear back from Brentnall for months.
Brentnall’s lab was having trouble with the palladin gene, which is huge and complex. So a technician from Brentnall’s lab came to work with Otey, staying in her house while they worked together. After a few more months, the collaboration paid off: Family X carried a mutation in the palladin gene that changed just one amino acid from a proline to a serine. All of the affected family members had the serine—the others had the proline. This was the first clue to what was causing pancreatic cancer in Family X, and it would eventually lead Otey to a possible tool for early diagnosis.
An unexpected discovery
Otey discovered palladin by accident in 1991. She was a postdoc at UNC studying the cell’s cytoskeleton. Her research focused on alpha-actinin, which can bundle and anchor filaments of actin, one of the main components of the cytoskeleton. Otey had an antibody that she thought recognized alpha-actinin, indicating when it was present in cells or tissue. It took four years before she figured out what it was actually recognizing: a new protein that binds to alpha-actinin.
Otey soon figured out that, like alpha-actinin, the new protein regulates actin and the cytoskeleton. That’s where she got the idea for the name. Because the protein was controlling the architecture of cells, Otey named it after the Italian Renaissance architect Andrea Palladio.
Before she could publish on palladin, Otey had to clone the gene that produces the protein. Cloning in this context means copying the gene from the genome into a form that can be sequenced and manipulated to study its function. This was in the days before the human and mouse genome projects; it took a long time to clone the palladin gene. But along the way, Otey began studying the function of the protein, eventually finding that it was activated by cells during wound healing. This was the first hint that palladin might be involved in cancer.
Otey says tumors are often described as wounds that don’t heal. And when tumor cells metastasize they can behave a lot like cells responding to an injury. So Otey began to look at palladin in breast cancer cells. Later, she got the call from Brentnall.
After a couple years of work, Otey and Brentnall published their discovery of the palladin mutation in Family X. Otey continued studying palladin in breast cancer cells, but knew she had to follow this lead on pancreatic cancer. The problem was, Otey didn’t know much about pancreatic cancer.
Rare but deadly
Pancreatic cancer is the fourth leading cause of cancer deaths, but it doesn’t have the visibility of the others in the top five, like breast cancer or prostate cancer. Patrick Swayze, who in 2009 died from pancreatic cancer twenty months after his diagnosis, helped raise the profile of the disease. But it still doesn’t get the research funding and attention of the more high-profile cancers. It’s not associated with a certain behavior, like lung cancer is. There’s no screening test. Only about 5 percent of patients live five years. Many only make it a few months.
The problem is that pancreatic cancer is almost never found early. The pancreas, which is responsible for controlling glucose levels in the blood and helping digest our food, is tucked away at the back of the abdomen, just beneath the stomach. It’s so buried that it’s hard for doctors to check during physical exams. Symptoms of pancreatic cancer, including back pain and weight loss, are vague and can be mistaken for other diseases.
Once pancreatic cancer is diagnosed, it is hard to treat. Chemotherapy and radiation are ineffective. Surgery is the only hope, but often the cancer has spread by the time it’s diagnosed, making it impossible to remove. Even when patients are eligible for surgery and surgeons think they have removed all of the cancer, only 25 percent of those patients live five more years.
A clinical-science partnership
Luckily for Otey, as she was trying to figure out how to direct her research, she met with a few other researchers on campus interested in pancreatic cancer. One was H. J. Kim, a surgical oncologist who specializes in pancreatic cancer. In addition to seeing patients and operating, Kim was studying tumor resistance to chemotherapy. He was immediately intrigued by Otey’s work.
Kim invited Otey to join him at a meeting of the local chapter of the Pancreatic Cancer Action Network. Kim was there to give a presentation, but it also gave Otey a chance to meet the people she was trying to help. One woman’s husband had died of pancreatic cancer—so had other members of his family, and his wife was terrified for her children. “It really raised my consciousness about the urgent need for more research in this area,” Otey says. “It motivated me to work on this.”
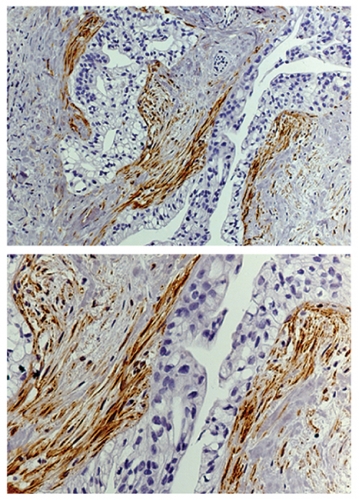
Together, Otey and Kim have found some surprising results. There are at least nine so-called isoforms of palladin; other labs had shown that one form is much more prevalent in pancreatic tumors than in normal tissue. But Otey and Kim were shocked to find that this isoform wasn’t in the cancer cells—it was in the cells surrounding the cancer.
These surrounding cells, called tumor-associated fibroblasts (TAFs), may hold the key to how tumors grow and metastasize, Otey says. “They also secrete a dense layer of collagen around the tumor that may be acting as a barrier to chemotherapy drugs, walling off the tumor.”
Kim says that pancreatic cancer is particularly resistant to chemotherapy and is known for a prevalence of TAFs. So palladin may be influencing both how the tumors spread and how they respond to treatment. Someday doctors may be able to use this knowledge to improve chemotherapy or slow the metastasis of pancreatic cancer.
But Otey is excited that another aspect of their work may benefit patients much sooner. The TAFs, and palladin along with them, show up very early in the disease. Using mice that were genetically engineered to develop pancreatic cancer, Otey found this isoform of palladin when the tumors were just beginning to form. This could help detect the disease earlier using a technique already in practice.
When a doctor sees a suspicious mass in the pancreas, she can use ultrasound to guide a needle to the mass. A pathologist has to look at the extracted tissue and determine if it’s cancerous, a challenging prospect. Instead, the pathologist could just stain the tissue with an antibody for this isoform of palladin. Because the TAFs surround the tumor, the needle would collect some of these cells along with the cancer cells. This isoform of palladin is specific to TAFs, allowing the doctor to rule out other diseases that can be mistaken for pancreatic cancer.
It will take clinical trials to prove that this process will work with needle biopsies from patients, but Otey and Kim got close: they could detect this isoform of palladin in needle samples from tumors that Kim had removed from his patients. And anything that improves early detection of pancreatic cancer is a welcome development, especially for families waiting for a diagnosis.
A tale of two approaches
Otey says their work is a perfect example of the different approaches of basic scientists and clinicians. “There are two completely different ways of doing science,” she says. “Basic scientists mostly try to unravel underlying mechanisms.” They want to understand how a disease works so doctors can know how to treat it. Clinical scientists care about mechanisms too. “But what they really care about,” Otey says, “is does this information tell us a better way to diagnose or treat this disease? And they care about it now.”
Otey and Kim may have found a new diagnostic tool for pancreatic cancer. But they also understand a little better how the disease works and possibly why it is so aggressive and so resistant to treatment. That’s the power of collaboration. “It has shifted my priorities,” Otey says. “I’m still a basic scientist and I still want to understand underlying mechanisms, but I’m constantly on the alert—is there something here that would help us immediately?”
Kim also makes it clear how much he depends on his collaborations. He spends one day a week seeing patients, and one or two days in the operating room. Whatever time is left is for research. That would never be enough to compete for grants and publications without collaborators such as Otey. But if Kim spent any less time in the operating room, he says, he would not be the kind of surgeon you would want operating on your family member. “You can’t turn that on and off,” he says.
Kim has helped expand their collaborations, including what he describes as “mini clinical trials” with human tumor samples grown in mice in the lab of fellow surgical oncologist Jen Jen Yeh. This could dramatically accelerate drug discovery.
But Kim and Otey are realistic about the pace of research. It’s been over a decade since Otey discovered palladin and six years since that call from Brentnall sparked Otey’s interest in pancreatic cancer.
In fact, they still don’t know exactly why the palladin mutation in Family X caused pancreatic cancer. Otey thinks it may not be affecting the cell’s architecture via actin, but palladin may be entering the nucleus and changing which genes are turned on. Otey and Kim will keep trying to figure out what palladin does and how pancreatic cancer works, but always with an eye to how they can help patients.
Alex Raines is an MD/PhD student studying neurobiology.
Carol Otey is the interim chair of the Department of Cell and Molecular Physiology in the School of Medicine. Hong Jin Kim is an associate professor of surgery in the Division of Surgical Oncology. Otey and Brentnall published their discovery of the mutation carried by Family X in PLoS Medicine in December 2006. Otey and Kim published their work on pancreatic cancer in April 2010 in PLoS One, and their work with breast cancer cells appeared in Oncogene in January 2009.
Watch more videos from UNC Health Care here.