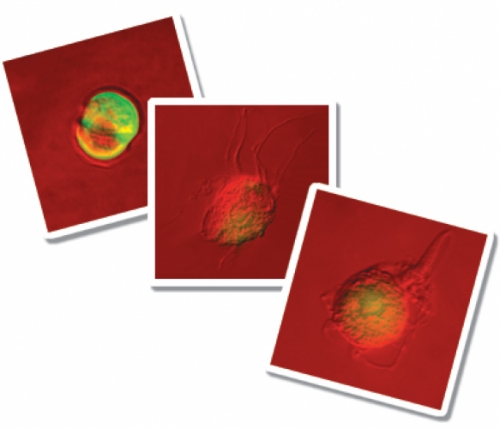
Remember Pfiesteria? In the late 1990s, North Carolina newspaper headlines warned that this single-celled water creature was killing fish. And maybe, they said, it could even harm you. Fishermen and tourists exposed to suspect waters, as well as researchers studying Pfiesteria in laboratories, had reported memory loss and other cognitive impairment.
The tiny creature seemed not only dangerous, but bizarre. The first scientific studies reported a 24-stage life cycle never before seen in this type of organism (a marine dinoflagellate). Pfiesteria’s shapeshifting included unusual amoeba and cyst stages. According to the reports, Pfiesteria showed its amoeba forms only in the presence of fish — when it was in killing mode. Many of these amoeba stages were reported to be toxic.
Now a team of researchers, having reexamined Pfiesteria using some new tools of medicine, are questioning the idea that the animal produces a toxin. In June 2002, research biologist Wayne Litaker and colleagues released results of their meticulous effort to precisely characterize Pfiesteria using a tool that has been around for only three years — peptide nucleic acid (PNA) probes. The team’s findings suggest that Pfiesteria isn’t so complex after all. The creature, the researchers say, shows a normal life cycle much like those of other dinoflagellates. The team published their findings in the June 20 issue of the Journal of Phycology, known as the leading journal on algae.
The project began with Pat Tester, plankton research team leader at the National Oceanic and Atmospheric Administration’s (NOAA) Center for Coastal Fisheries and Habitat Research in Beaufort, N.C. She had originally studied Pfiesteria in collaboration with N.C. State University scientist Joann Burkholder, who first identified Pfiesteria and observed the unusual life cycles. Tester began working on the organism on her own after discovering that there were many look-alike species living in estuary waters with Pfiesteria, which made it impossible to measure. She brought Litaker on board to develop molecular tests to definitively detect the creature.
For years, Litaker has worked in two different disciplines and in two different towns. Until recently he spent part of his week working for the Program on Molecular Biology and Biotechnology at Carolina, organizing and teaching two-week, hands-on training sessions in molecular biology techniques. He spent the rest of his time as a research biologist at NOAA’s Beaufort research center. That’s a lot of traveling and juggling, but Litaker calls it fun. “I’m one of those people who gets paid to do what they love to do,” he says. “You’re welcome to come and play in my lab anytime.”
Constantly fidgeting, Litaker explains his favorite toys — the molecular biology techniques he has spent years teaching — and how he and his colleagues applied them to marine biology.
The team started by carefully isolating the creature — they wanted to make sure they were studying Pfiesteria and nothing else. They started pure cultures of Pfiesteria from different sources, including water from North Carolina’s Pamlico River, where Pfiesteria was reported to be killing fish. Using a capillary tube that they melted into a fine, hollow thread, the researchers drew up the cultures one cell at a time. They cleaned the cell by transferring it into a new drop of sterile media and then drew the cell up into another pipette. They repeated this process until they were sure they had isolated a single cell. Now their Pfiesteria culture contained only two things — Pfiesteria and a species of algae known as Rhodomonas, which the researchers added as Pfiesteria’s food.
Next the team observed their cultures — in tanks all alone, in tanks with fish, in tanks with algae. They waited and waited to see the 24 life stages that other researchers had attributed to Pfiesteria. “Two years — nothing,” Litaker says. The researchers used video microscopy to record Pfiesteria’s normal dinoflagellate activities — the creature swam around using flagella, and when it had eaten enough algae, it dropped to the bottom and temporarily formed a round cyst. Some forms also went through mitosis — cell division or asexual reproduction — while others reproduced sexually.
If they hadn’t before, the researchers began to doubt the 24-stage life cycle. But how could they get to know this creature? Pfiesteria is notoriously hard to distinguish from other species with light microscopy and other techniques. Litaker’s team decided to bring out their medical tools — molecular probes. “These probes work because no matter what life stage your critter is in, the DNA stays the same,” Litaker says.
To make such a probe, scientists sequence the DNA of a creature until they find a piece of DNA that is unique to that organism. Litaker’s group sequenced a large segment of DNA from Pfiesteria and from a number of related species until they found a unique piece of Pfiesteria DNA. Instead of DNA probes, the team made PNA probes — synthetic cousins of DNA probes that work the same way but bind tighter and longer and aren’t easily degraded.
long with their high-tech tools, the researchers used some old-fashioned caution. They had observed, even before they added Pfiesteria, that their fish tanks already contained some amoebas — contaminants that came in on the fish. It’s nearly impossible to get a “clean fish,” Litaker says, because the chemicals that will kill bacteria, protozoa, and amoebas on fish would also destroy the fish’s skin cells. But, in its toxic stages, Pfiesteria was reported to take on the form of an amoeba. So, how do you distinguish between the run-of-the-mill amoebas and Pfiesteria amoebas? By being systematic. Before the researchers added Pfiesteria to their fish tanks, they took the time to culture the amoebas and other organisms already present and determine their DNA sequences.
Using these sequences and the ones from the Pfiesteria, the researchers developed two very specific probes. One was made to bind only to a unique stretch of Pfiesteria DNA. The second probe would bind to a unique piece of DNA in true amoeba cells. The researchers labeled the probes with fluorescent material so that when the probes bound to their target pieces of DNA, they would fluoresce — glow when illuminated with a specific wavelength of light.
But the team had to be careful to choose the right type of fluorescent label. Many amoebas will, all by themselves, autofluoresce — they will naturally give off a fluorescent glow when exposed to certain wavelengths of light. When testing some run-of-the-mill amoebas, the researchers found that the amoebas autofluoresced under the same light wavelengths as the fluorescent molecules commonly used to label DNA probes. “So we were careful to label all our Pfiesteria probes out in the far red spectrum, where the amoebas don’t just glow,” Litaker says.
When the scientists applied their unique Pfiesteria probe to the familiar dinoflagellate life forms from each of their Pfiesteria cultures, the probes tested positive — they glowed. But when the researchers applied the Pfiesteria probes to the amoebas from the tanks where Pfiesteria was killing fish, the amoebas tested negative.
But the team didn’t stop there. They tested the amoebas from the fish-killing tanks with the amoeba-specific probe. These amoebas tested positive only with this probe. This test confirmed that these were plain old amoebas — not Pfiesteria.
Other tests confirmed that the probes were working properly. For instance, to eliminate the possibility that some of the cells were impermeable to the PNA probes, the researchers used a commercially available PNA probe that is designed to bind to all cells that are not bacteria. This probe tested positive with the Pfiesteria and with the amoebas. This test proved that the PNA probes could get inside the cells just fine. But the amoebas wouldn’t respond to the Pfiesteria probe because they just didn’t contain Pfiesteria DNA. “These were not Pfiesteria amoebas,” Litaker says.
“We saw a lot of things that could be confused for all the previously reported transformations,” Litaker says. “But now we have the technology to determine for sure that the amoebas are not Pfiesteria.”
he Pfiesteria did kill Litaker’s fish. “They appear to do it by direct contact,” he says. His team observed Pfiesteria extend a small, enzyme-laden feeding tube, called a peduncle, into the skin of fish and “suck the guts out” of the fish’s skin cells. “Our observations were consistent with the fish dying from thousands and thousands of Pfiesteria cells feeding on the fish’s skin, and not necessarily due to a toxin,” Litaker says. “Though it’s possible that a toxin could be produced in the tanks, current experiments cannot distinguish if those toxins are coming from Pfiesteria, the many contaminating organisms in the tanks, or some interaction between those organisms that may or may not involve Pfiesteria.” So there’s no way to attribute such a toxin solely to Pfiesteria without further study.
“We’re arguing that we have to be really careful about ascribing toxicity to any life stage of Pfiesteria because there are a lot of unrelated organisms in these tanks,” Litaker says. “The question of toxicity needs to be reexamined.”
What about the fish kills seen in North Carolina’s waters? Some fish lesions could be caused by Pfiesteria, Litaker says. But other researchers, including Edward Noga, one of Litaker’s coauthors and a professor at the College of Veterinary Medicine at N.C. State University, have reported that as many as 40 different causes can produce fish lesions. And, in natural bodies of water, Pfiesteria has not been found in high numbers, possibly because tiny protozoa called ciliates feed on Pfiesteria. “The fish tanks allow an artificially high density of Pfiesteria to exist,” Litaker says. “If there’s a lion every three thousand miles, you’re not likely to get eaten by a lion. But if you go into that lion’s den, you’re gonna be lunch. Extrapolating from the tanks to the real world should be done with extreme caution.”
Though this research suggests that Pfiesteria is not a serious threat, that doesn’t mean that we should stop watching over the waters. “There are major harmful algal bloom problems worldwide, and they get worse every year,” Litaker says. Several species of algae such as Karenia brevis and blue-green algae produce potent toxins that can be passed on to humans who eat infected fish. Litaker is now working to apply molecular techniques to learn more about the life cycles of these toxin-producing algae in hopes of developing methods of detecting blooms and fish infections. He says, “We’re taking the best tools that we can get out of medical research and applying them to a public health problem.”
In addition to his work in the UNC School of Medicine, Litaker is an adjunct research assistant professor of biology at Carolina. Other authors of the study are Victoria Madden, research analyst in pathology and lab medicine at Carolina, and Mark Vandersea and Steven Kibler, both of NOAA. NOAA funded the study.
tracking the health of workers on the water
A study by Carolina epidemiologists and other scientists may soon provide more information about the dangers of working in East Coast estuaries. But it won’t provide a final answer as to whether Pfiesteria causes health problems.
Christine Moe, David Savitz, Paula Bell, and David Weber, all from the Department of Epidemiology in the School of Public Health, and colleagues are conducting systematic studies of exposure to estuary waters in North Carolina, in parallel with other studies in Virginia and Maryland. Researchers for each site recruited groups of 118-238 subjects who work at least eight hours a week in coastal waters. Subjects received physical and neurological exams and were reexamined every six months, or more often if they reported working where a fish kill had occurred. Researchers also periodically interviewed subjects by telephone or had them keep diaries.
The team is now analyzing the data and hopes to release a final report in about a year, says Savitz, chair of epidemiology. The researchers were mainly looking for evidence of a specific set of symptoms reported to be associated with Pfiesteria and known as “possible estuary-associated syndrome.” A report based on two years of data and released in October 2001 stated that the researchers had not found evidence of the full-blown syndrome, which includes numbness and tingling in the hands and feet, difficulties with concentration and memory, and personality changes. But some workers may have experienced less severe symptoms. And during the time the data were collected, there were no fish kills in North Carolina clearly documented to have been caused by Pfiesteria.
The study will provide some information about correlation between health problems and patterns of exposure to water. “But everything related to Pfiesteria exposure really becomes an inference,” Savitz says. “For example, some people work in estuaries or parts of estuaries where Pfiesteria has been identified in the past. So we might infer that those workers are at greater risk of exposure to Pfiesteria than those who work where Pfiesteria hasn’t been found.”
But the study will not be able to pinpoint whether Pfiesteria has caused specific instances of health problems. “Was there Pfiesteria in the water at the relevant time, or was there exposure to the toxin that is potentially produced by the Pfiesteria — that’s a level of detail that we won’t have,” Savitz says. “I think we’re doing what can be done to address the concern, but we can’t claim that the study will be in any way definitive with respect to Pfiesteria exposure and health effects.”