We’ve all heard the phrase “Just Say No” to drugs. But Mark Schoenfisch is telling people to “Just Say NO” to drugs. What’s the difference?
To Schoenfisch and other chemists, NO means nitric oxide—a gas not to be confused with nitrous oxide, or laughing gas. Schoenfisch thought carefully before diving into NO research. How could something that comes out of car exhaust and cigarette smoke have healing properties?
But NO does have a big hand in maintaining the body’s critical functions, and it was even named “Molecule of the Year” by Science in 1992. That places it alongside such scientific milestones as synthetic diamonds (1990) and Dolly the Sheep (1997).
NO ain’t new. Chemists have been probing the role of nitric oxide in human physiology for half a century, and have discovered that it is released endogenously—by the body itself—in small quantities. It’s a veritable Swiss Army knife of vital tools, yet it’s still smaller than a molecule of oxygen.
It’s a neurotransmitter. It releases neurotransmitters. It’s necessary for memory and learning. It helps modulate digestion, respiration, and almost everything in between.
These myriad roles arise from nitric oxide’s capability as a vasorelaxant—sort of a massage therapist for cells. The walls of healthy arteries release nitric oxide, which relaxes the surrounding muscle. This can help open blood vessels to vital blood flow, regulating blood pressure and flow all over the body. Too little nitric oxide can result in platelet adhesion and blood clotting, while too much could potentially cause incessant bleeding.
This relaxation property is likewise responsible for elevated NO levels in the uterine walls of full-term pregnant women; the NO helps thwart premature contractions. In men, NO promotes the blood flow that enables intercourse.
Scores of people have benefited from the relaxing quality of externally delivered NO, too. Nitroglycerine, essentially a deluge of nitric oxide, opens blocked arteries and, by allowing blood flow, has saved the lives of millions of heart-attack patients.
NO also promotes wound healing and kills bacteria and fungi. Genetically mutated mice lacking NO synthase, the enzyme that triggers NO production, have wounds that just don’t heal. No enzyme, no NO, no healing. This is because NO mediates multiple processes in the wound-healing cascade—“re-epithelialization, wound closure, tissue granulation, tissue remodeling, and scar-tissue prevention,” says Nathan Stasko, a fifth-year graduate student at UNC who has studied nitric oxide-based analgesics.
Wound healing can be further impeded by bacterial and fungal growth, so not only is NO necessary for repair itself, but also preventing the bacterial colonization that can encumber any one of the components of the cascade. “So if you treat the bacteria,” Stasko says, “you heal the wound.”
What part of NO doesn’t he understand?
But it appears that having NO-releasing properties isn’t enough, and even though nitric oxide has all these valuable properties, NO drugs have yet to flood the market. Nitroglycerine is the only NO-releasing product doctors use, due to one problem (that ironically saves heart-attack patients)—the unrestrained barrage of the gas into the body. Unfortunately, it goes anywhere and, like any molecule, can be toxic in uncontrolled amounts. No current devices use NO’s properties because no one has been able to safely store and deliver it.
“It all comes down to the innate difficulty of storing NO and controlling its release,” Schoenfisch says. “People are after that. There are hundreds of patents on hundreds of NO donors—but none has led to the development of a useful product because of the issue of storage and controlled release.”
Complicating storage even more is the fact that different processes are mediated by different amounts of nitric oxide. At low concentrations, says postdoctoral student Jae Ho Shin, “NO is involved in improving wound healing. However, if we are interested in killing bacteria, we need a much higher concentration of NO.” This is why Schoenfisch’s team is constantly pushing the envelope to develop new materials that can hold more and more of the molecule. And it’s paid off—they’ve synthesized materials that store more nitric oxide than any others in the world.
Getting to know NO
Schoenfisch’s involvement in NO research began with a postdoctoral position at the University of Michigan. With his advisor, Mark Meyerhoff, he worked on exploiting nitric oxide’s power to reduce platelet adhesion. Current methods for measuring blood oxygen levels, for example, involve injecting blood into large gas analyzers. The consequent time lag between the output reading and a patient’s actual blood oxygen level can be damaging: in a critical-care situation, blood-gas sensors implanted directly into arteries could be lifesaving. Unfortunately, a foreign material embedded in the body is a welcoming surface for platelet and bacterial adhesion, leading to rejection and infection. Schoenfisch was part of the first group to publish animal studies on an intravascular gas and electrolyte sensor made of a material that gradually releases low levels of nitric oxide to prevent such biofouling.
Since then, Schoenfisch has ventured into other NO-releasing biomaterials, beginning with the design of NO-releasing glucose sensors that could be implanted subcutaneously, giving constant, accurate readings of blood glucose levels without the need for inconvenient hourly assays.
In 2001, when Schoenfisch initially focused his efforts on implant-related infections, he expected to take advantage of NO’s promiscuous role in fighting infection. “That’s the beauty of NO,” says Shin, who has been synthesizing NO-containing biomaterials in the Schoenfisch lab for the past six years. With all of NO’s versatility, it’s possible to design a therapeutic that uses all its efficacious properties in concert. “Typically, different drug molecules are employed to prevent platelet adhesion, kill bacteria, and promote wound healing at the implantation site,” Shin says. But with NO, they can simply reach into its mixed bag of tricks and achieve all three.
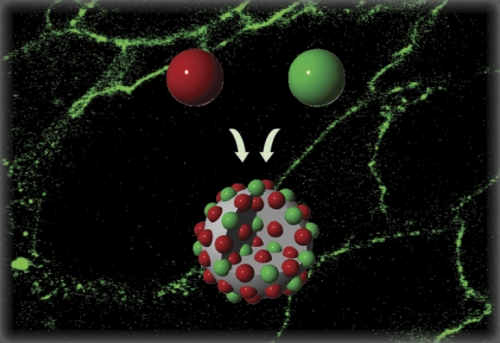
But that leaves the problem of NO storage and delivery, and one way Schoenfisch manages this is by synthesizing NO-releasing nanoparticles with controlled storage and release of nitric oxide. A nanoparticle is the bridge between a molecule and bulk material: the middle kid who’s sort of different, more capricious than the others. Changing the size of the particle—typically by simple changes in reaction conditions—actually alters its physical and physiological properties.
First Schoenfisch’s group made gold clusters, and then dendrimers, which resemble the pattern on a cracked windshield. But their most recent silicon-based nanoparticles are what have shattered records for NO-storing capacity over any other material. These materials are made by exposing a simple organic molecule—the donor—to a high pressure of NO gas, which locks nitric oxide within each molecule. Next, these small NO-trapped molecules called nonoates are attached to a silica scaffold—essentially the stuff ceramics are made of.
The size of the resultant nanoparticle, and thus the amount of NO trapped within, can be easily increased or decreased by changing the reaction medium, sequence, concentration, and size of the silicon precursor. Shin has been able to make particles ranging from twenty to six hundred nanometers in size, which he believes could make the difference when targeting cancer cells that have large holes in their cell membranes from tumor growth and stretching. He’s investigating the selectivity of the large silica-based scaffolds against ovarian cancer cells, and has already found that not only are the NO nanoparticles too large to cross into healthy cells, but once they have penetrated malignant cells, they remain inside for much longer. This is their first attempt at size-selective targeting, Shin says, “and there is a lot more potential and possibility in that area.”
And Schoenfisch can control the delivery of NO just as easily as he can its storage capacity, by varying the type of donor molecule that resides on each nanoparticle. The way NO is released in the body depends on the structure of the donor, and Schoenfisch believes he can exploit the contrasting mechanisms in targeting. For example, a donor designed to release NO as a swift eruption should be more effective in killing bacteria, while a donor that only releases NO in response to distinct triggers in the body may be better at mediating biological processes over a long time.
So not only is Schoenfisch able to increase the trunk space of the materials, but Stasko says that their recent investigations have resulted in nanoparticles that “finely tune the rate of NO release.”
The future of NO
Stasko and Schoenfisch have founded a corporation based on their patented NO-releasing materials. NOVAN (Nitric Oxide Vehicles and Nanotechnology) may take off into a wide variety of therapeutic areas, Stasko says, but they are initially concentrating on healing topical wounds such as burns. “Burns have an extremely high rate of infection, and you also have a good deal of scarring that occurs. Commonly employed burn antibacterials don’t include a second wound-healing component,” he says. But with all the evidence pointing towards the synergic properties of NO, they are confident that their products will do both.
Right now, the most exciting strategy available for infection is polymeric silver release, which, like nitric oxide, exhibits analgesic effects. The problem is that bacteria are developing resistance to the silver influx.
“But NO is going to be very difficult to develop resistance toward,” Schoenfisch says, “because it is known to diffuse rapidly through polymers and cell membranes.” And whereas antibiotics are typically administered systemically, promoting resistance all over the body, the localized delivery of NO will further discourage resistance.
“Ultimately we would like to develop lots of different NO-releasing technologies and license them for different applications: wound healing, anti-infection, and biofilms,” Schoenfisch says. The team hopes they can then venture into bigger territories such as ischemia and cancer.
Schoenfisch also wants to further explore selective delivery in the cancer arena. His team has already begun modifying their NO-releasing nanoparticles with folic acid. As ovarian cancer cells express more folate receptors on their surfaces than non-tumor cells, an ideal drug would inundate the surface of a cancer cell before a healthy cell and deliver NO selectively.
But still, with no NO on the market today, Stasko says, “investors are very leery because they can’t compare our technology to other NO-releasing products—the safety, the efficacy, the cost. We’re pushing the field, and that’s a challenge.” The upside is that they’re armed with the results of exhaustive toxicity studies, which reveal that these nonoates should be more appropriately called yes-yes-ates.
Danielle Jacobs was a student who formerly contributed to Endeavors.
Mark Schoenfisch is an associate professor of bioanalytical chemistry in the College of Arts and Sciences. His research on nitric oxide-releasing nanoparticles is funded by the National Institutes of Health and the Carolina Center of Cancer Nanotechnology Excellence, a National Cancer Institute-funded collaboration between UNC and the Lineberger Comprehensive Cancer Center.