Joy Cook regained consciousness, glanced around, and remembered that she was in a hospital bed. It wasn’t an unfamiliar setting. She was almost twenty-two years old and had been diagnosed with cystic fibrosis when she was seven. Ever since, she’d had regular hospital stays, each lasting three or four days. But two things about this situation frightened her: the doctors swarming around her were in a panic, and she felt worse than she ever had in her life.
She looked at her mother across the room and could see that she’d been crying. They’ve talked to my mom, Joy thought. They told her I’m dying. I’m pretty sure I’m dying.
For several days leading up to this, Joy had lain in her hospital bed watching the numbers on the screen next to her — heart rate, respiration rate, oxygen saturation — jumping well out of the normal range. She tried to control them by taking deep breaths. It didn’t work.
One of the doctors saw that Joy was awake and stood over her. “We need to transplant you,” the doctor said. “Can we do that? Is that okay?”
Joy couldn’t speak, so she nodded.
She woke again later in the day and the chief transplant surgeon had come into her room. “You’re officially on the list,” Thomas Egan told her. “We’ll keep you as healthy as we can, but this is going to be a very hard surgery.”
Joy spent twelve hours on the operating table, including a session of emergency pulmonary bypass. She surprised everyone, though, by making a fast recovery. She went home just fifteen days later, able to walk and breathe on her own, the draining tubes finally removed from her chest. She spent the next semester — what was supposed to be the last of her senior year at Carolina — in recovery. But soon Joy was back in class, and graduated just after her next birthday.
“Before my transplant, even when I was at my healthiest, I still never felt as good as I do now,” Joy says today. “I just never knew what it was like to be really healthy. I don’t know if I’ve ever had lung function as ridiculously high as I do now.”
In May 2005, just a year and a half before Joy’s surgery, Egan had helped the Organ Procurement and Tissue Network (OPTN) make huge changes to the U.S. lung allocation system. If Joy had gotten sick before the system changed, Egan says, they never could have gotten her name to the top of the transplant list in time to save her, no matter how ill she might have been. Now Egan’s working on new research that will help patients like Joy in the coming years. He wants to make sure there are enough lungs to go around.
A dying system
Before the changes to OPTN in 2005, the U.S. lung allocation system was based on waiting times and geography, Egan says. The average waiting time was about a year and a half, and began the second a patient was put on the list. Even so, hundreds of patients died long before their names reached the top. And even for patients at the top of the list, the organs had to come from a donor close by.
For example, Egan says, when a set of lungs became available for transplant, it was offered first within the hospital where the donor had died, then to hospitals within the jurisdiction of the local organ procurement organization (OPO), and then within a radius of five hundred miles.
The arrangement created plenty of opportunities to cheat the system. Doctors wanted to champion for their own patients, Egan says, and if there was a way they could manipulate things in their patients’ favor, they would do it. Some would list patients long before a transplant was necessary, or even leave names of patients who had died on the waiting list, so that when the local OPO called to offer an organ, the hospital could quickly pull up the name of a living patient who could use it.
“I had some serious issues with that allocation system,” Egan says. So when OPTN asked him to chair a committee charged with proposing a fair, efficient system for lung allocations, he jumped at the chance. “My goal was to come up with a completely data-driven system,” he says. “One that couldn’t be manipulated.”
The committee set out to create a complex algorithm that would assign each patient on the list a score based on severity of illness, chances of dying without a transplant, and probability of survival after a transplant. It took years of setbacks and negotiations, Egan says. When the committee finally finished the algorithm and ran it through a simulator, it predicted that the number of patients who died on the waiting list would be reduced by 10 to 15 percent.
In fact, the new model reduced deaths by half. The number of deaths on the waiting list went from 500 a year to 250, and the number of patients getting transplants went from 1,000 to 1,400 in one year. In 2009 the number of transplants reached 1,500. Under the new model, patients who are in immediate danger of dying are offered organs. And now, OPOs don’t have to spend nearly as much time searching for recipients, Egan says. Before 2005, OPOs had to make about eleven phone calls before finding a match for a donor’s lungs; today, the median number of calls is three.
Lung in a bag

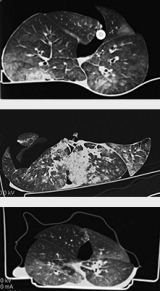
Egan uses CT scans such as these to detect pneumonia and other hidden conditions that render lungs unsuitable for transplant. The top scan shows a donor lung with bilateral pneumonia. In the middle scan, he found some patch infiltrate, probably from pneumonia. And the lighter color in the bottom scan shows a build-up of fluid known as dependent edema.
Click to read photo caption.
Lung disease is the fourth-leading cause of death in the United States, and it kills some two hundred thousand Americans every year. Most of those deaths are caused by emphysema, pulmonary fibrosis, cystic fibrosis, and pulmonary hypertension, the diseases that most commonly lead to lung transplants. While there are usually only about eighteen hundred people on the lung transplant list, Egan says, there are thousands more whose names never make it to the roster.
This is because doctors tend to list only young patients, who have a higher chance for post-transplant survival, Egan says. Many patients with emphysema are in their seventies or eighties, and putting older people on the transplant list is incredibly controversial. “Is it ethical to transplant a seventy-year-old if you’ve got a twenty-two-year-old waiting for lungs?” he asks. “I don’t have the answer to that. I just know that there aren’t enough lungs.”
Now Egan and his team are using a $1.4 million grant from the National Institutes of Health to test and perfect a new technology that could drastically increase the number of lungs suitable for transplant. Egan thinks it could also help surgeons to draw from a donor pool that no one has ever before considered.
Only about 20 percent of lungs in the pool of U.S. organ donors are suitable for transplant, Egan says. Most lungs come from donors who have suffered head injuries and have later been declared brain dead. The initial brain injury sometimes causes simultaneous vomiting and gasping, which pulls fluid down through the airway. After a few days on a hospital ventilator, that person has developed pneumonia, and his lungs are not transplantable, Egan says. But the reason that most of those lungs aren’t eligible for transplant is called ischemia reperfusion injury, which happens when a patient’s blood flow stops and then starts again — for example, when doctors put a patient on life support.
A solution to the lung shortage first occurred to Egan one day in the mid-1980s. He was reading a journal article about bronchial epithelial cell cultures when a small note at the bottom of the page caught his attention. It read: Human bronchial epithelial cells were retrieved from morgue specimens in the usual manner.
“Morgue specimens,” Egan says. “You can’t grow cells that are dead. You can only grow cells that are alive.”
After some investigation, Egan found that lung cells are different from the cells in other organs: lung cells don’t depend on a heart pumping blood to get oxygen, he says, because they can get oxygen from the air. This means that unlike other organs, lungs don’t begin to decompose immediately after a person dies. “We know that when the heart stops, your brain dies within minutes, which is why we do CPR,” Egan says. “Your heart dies within minutes. Your liver dies within minutes. But your lungs — here’s this guy in the morgue, hours after death, and yet you can still grow his cells in culture.”
Egan’s thoughts kept going back to the article over the next few years. And he asked himself again and again: What if surgeons could have access to the organs of people who died outside of hospitals? Of “unconventional donors” who died at home, on the road, or in work accidents? “I thought at first that this idea was so crazy, someone else must have thought of it,” he says. But nobody had.
“There are three quarters of a million sudden deaths in the United States every year,” he says. “If we could get our hands on just the youngest 5 percent, that’s more than thirty-five thousand donors. And since many of the patients we could transplant would need single lungs, we could be doing upwards of fifty thousand transplants a year, easily.”
But even after Egan had proven in the lab that he could successfully transplant lungs from dogs that had been dead for up to four hours, his colleagues around the country were still shocked and doubtful of the idea. It’s taken years of battling with funding agencies and skeptical colleagues to get to this testing stage, Egan says. But now he’s found the technique that could allow him to carry out his unconventional idea.
Using a method called ex vivo lung perfusion (ex vivo means outside the body), Egan can treat donor lungs that have been injured by brain death or that have started to build up fluid during the hours after a donor’s death — and restore them to a transplantable state. A video he created to illustrate his technique shows human lungs in a plastic bag, breathing through a ventilator. Five liters of dark-red, deoxygenated blood circulate throughout the lungs every minute in a process called perfusion. Bright-red, oxygenated blood filters away from the lungs through another tube.
Egan points at the bag on the screen. “This is going on inside you right now,” he says. “That’s how much blood is going through your lungs.”
It’ll take time to perfect the technique and for it to take hold in the medical community, Egan says — but ex vivo lung perfusion by itself could double the number of lungs from conventional donors for transplant in the United States. “Although, if we only double the number in the United States from fourteen hundred to twenty-eight hundred, that’s still not anywhere near the number that we need,” he says. The real numbers will come from unconventional donors — people who die suddenly, rather than days or weeks after brain death.
Of course, getting access to donors who die in the field is going to be difficult, Egan says. EMS personnel, medical examiners, and emergency room personnel will have to be retrained, and outdated laws in some states about brain death in organ donors would have to change. “But it’s a challenge we can deal with,” he says.
Happy Lungiversary

Photo by Jason Smith. ©2010 Endeavors magazine.
After Joy’s surgery, her doctors asked her if she’d like to donate her lungs to the hospital for their research. “That would be awesome!” she said.

Click to read photo caption. Photo by Jason Smith. ©2010 Endeavors magazine.
In December of 2009, Joy Cook celebrated the third anniversary of her transplant. Her friends gave her a homemade cake, the words “Happy Lungiversary” spelled out in icing and lit by a candle in the shape of a 3. She has a job she loves in UNC’s biostatistics department, managing data from clinical trials. She can take trips without hauling her bulky therapy vest along with her. And her parents, who still live in Joy’s hometown outside of Boone, North Carolina, don’t worry so much about her anymore. “I’m totally self-sufficient,” Joy says. “I just bought a house. When I told my dad I was thinking about buying a house, his response was, ‘Well, you can be in debt like all the rest of us!’ Before he might have said, ‘Do you think you can afford that with all your medicine?’”
Egan’s own health problems and several back surgeries left him disabled soon after Joy’s transplant, and he had to stop his work at the operating table after some thirty years as a surgeon. Photos of his patients still crowd the tops of his office bookshelves, though, which is part of the reason he still comes to the lab every day. He sees Joy occasionally when she’s in for a checkup, and they chat about where his research is going.
“I think it’s fascinating — I read everything I can find about it,” Joy says. “It’s research like they do here at UNC that saved my life and the lives of other people I’ve met since this. It might look like it’s really high-risk on paper, but in the end, you’re literally saving lives. That seems pretty legit to me.”







