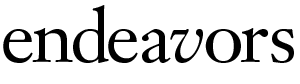Ted Salmon explores the bustling inner lives of dividing cells with the help of high-tech tools. His instruments help him view - and rearrange - the dynamic “nanomachines” inside, as he learns about the many forces that move chromosomes.
In textbooks, there is order. Cell division is precise and scheduled, with chromosomes marching in formation.
Ted Salmon, professor of biology, knows better. He starts his VCR, and the television screen fills with writhing, fine-spun tendrils - a silhouette of Medusa’s hair.
“Isn’t it great?” exclaims Salmon, watching the tendrils reach out and retreat. “That’s part of the fun I have: being able to see these ‘biological nano-machines’ in living cells.”
We’ve been looking at the inner workings of a newt cell magnified about ten thousand times. Each strand of Medusa’s hair is actually a protein chain, called a “microtubule,” that is growing and shrinking.
Visualizing such things is how Salmon made his mark in cell biology. He is an engineer-turned-biologist who links image-processing electronics with microscopes, peering deep into dividing cells. His video microscopy systems reveal the bustling, sometimes irregular activities within a live cell - qualities that static snapshots only imply.
“It’s easy to answer the question, ‘Do the cells get to this stage? Do the cells divide?’ Ordinary slides can show you that,” Salmon says. “But when and how do they get there? How orderly is the process? Those are the questions video microscopy can answer.”
And those are the questions that have shaped biologists’ understanding of mitosis, the cell’s way of reproducing, and of why the process sometimes goes awry - causing diseases like Down syndrome or cancer.
Over the past 20 years, Salmon’s studies of mitosis have focused on microtubules, the specialized “rails” that chromosomes move along, and on kinetochores, patches on the chromosomes where microtubules attach. He and his students have been able to visualize the dynamic interactions of structures like these since 1985, when Salmon built his first system, the VE-DIC (video-enhanced digital interference contrast) microscope.
The VE-DIC system is sensitive enough to display a single microtubule in nerve and tissue cells. Actually, the microscope can’t focus on a microtubule, which has a diameter four thousand times smaller than that of a human hair. Instead, specialized electronics detect the tiny amount of light scattered by each microtubule and produce a picture. This technology was developed in the early 1980s by two groups of researchers working independently. One group was headed by Salmon’s former graduate advisor, Shinya Inoué, then a biology professor at the University of Pennsylvania.
Salmon and his students used VE-DIC microscopy to study how microtubules connect to chromosomes and how chromosomes move along microtubules. They helped confirm a prediction that probability plays a role in chromosome movement, influencing the growth of microtubules toward chromosomes (see “Casting for Chromosomes,” above) and the motion of chromosomes once they are connected. As these processes became clearer, Salmon wanted to learn more about these microtubule “rails.” How strong are they? How flexible?
These questions led him to another tool, the laser optical trap, which precisely manipulates cellular components. Like a tiny pair of tweezers, the optical trap’s light beam grasps and carries a single microtubule to another object, such as a kinetochore, held stationary in the trap. This deliberate movement overcomes the thermal motion, generated simply by warmth, that randomly scatters objects in a cell. Salmon has begun this work, using a laser optical trap in Mike Sheetz’s lab at Duke University, and has been awarded $80,000 from the National Institutes of Health to build his own.
As Salmon has investigated the structural properties of microtubules, he has also studied their growth. Microtubule fibers grow and shorten at both ends, even when chromosomes are attached, and this helps move the chromosomes. To determine how much motion each process contributes, Salmon needed to mark the microtubules and to observe the marks and the whole array of microtubules simultaneously. He needed a multi-mode digital imaging microscope, a system he designed and built two years ago.
The multimode digital microscope generates both traditional DIC pictures and images of fluorescent molecules, which serve as colored beacons for locating specific proteins or for tracking marks on microtubules. A CCD (charge-coupled device) - a digital camera originally designed for astronomy instead of the microcosm of the cell - captures the images and measures the amount of light collected. The images can be superimposed, showing the relative movements of the structures. Changes in light intensity indicate when proteins have been broken down, produced, or scattered.
Despite the new microscope’s utility for some projects, Salmon hasn’t abandoned his old stand-by, VE-DIC. Recently, it allowed two researchers in the Department of Biology, Kerry Bloom and Elaine Yeh, to discover a new “checkpoint” during yeast mitosis. Certain checkpoints - pauses during mitosis when the cell makes sure crucial steps are complete - have been known for years. But the one found by Bloom and Yeh occurs at a different stage of mitosis, much later than the others.
VE-DIC microscopy is also non-invasive. More recent techniques such as fluorescent tagging add foreign materials, some of them toxic, to the cell.
“You truly can be an observer with VE-DIC,” Salmon says, “and watch the wonderful activity inside a live cell without changing a thing.”
When Cell Division Goes Awry
Down syndrome is one of the most common diseases caused by errors in mitosis or in meiosis, the closely related process of cell division in sperm and egg cells.
The disease occurs in one out of every 600 infants and is caused by the presence of three, instead of two, copies of chromosome 21. Every month, one to three newborns with Down syndrome are referred to UNC Hospitals, says Cynthia Powell, assistant professor of pediatrics.
In 95 percent of Down syndrome patients the three copies of chromosome 21 are separate, as chromosomes normally are. This condition, called “trisomy 21,” arises spontaneously rather than being inherited. However, having one child with trisomy 21 increases the chance of having another with the condition to 1-2 percent. The risk also increases as a woman ages.
Down syndrome also results from translocations, meaning that the third copy of chromosome 21 is attached to another chromosome. This condition, which accounts for 3 percent of the cases, may occur spontaneously or may be inherited from an unaffected parent. If it is inherited, the chance that subsequent children also will have a translocation ranges from 5 percent to 100 percent, depending on the type of translocation.
Having three sex chromosomes is also possible. These trisomies usually are less severe than Down syndrome, though they may cause learning disabilities and problems with socialization. However, the presence of three copies of most other chromosomes is lethal.
Although the cause of trisomies is unknown, some researchers suspect that most problems occur in eggs during meiosis. Because eggs are produced from infancy and may remain in a female for as many as 40 years, some eggs may have a prolonged risk of exposure to factors that can disrupt cell division.
Salmon’s work has been supported by the National Institutes of Health for 20 years. He is a member of the Department of Biology in the College of Arts and Sciences.