At the start of The Empire Strikes Back, a robotic probe lands on the ice planet Hoth, rises from the snow, and searches for the Rebellion’s hideout. The long-limbed, metallic probe finds a specific structure and determines that it could be a field generator. Darth Vader orders the attack.
Now, put yourself in Vader’s boots for a moment. He had long considered the Rebellion a cancer, a blight on the face of the galaxy. If he could have, he’d have blown the entire planet of Hoth to smithereens. Alas, the Empire’s Death Star, which had a superlaser capable of such a task, had been destroyed in the previous Star Wars movie. So Lord Vader was forced to use a more precise method: he ordered a ground assault.
Okay, stay with me here.
Doctors want to help patients as best they can. Unfortunately, a lot of pharmaceuticals, such as chemotherapies, are too much like the Death Star’s laser beam of annihilation and too little like a narrow assault on specific cancer cells. Some treatments wreak havoc throughout the body. And sometimes the drugs don’t work well enough against cancer cells or tumors.
One reason for this is that a lot of drugs, especially cancer drugs, are inhibitors; they prevent some cells from making certain proteins. When those proteins aren’t expressed, cancer cells can’t divide. Inhibitors, though, don’t work perfectly. Many cancers and other diseases find ways to overcome the suppression. And because inhibitors are not selective, they don’t just squash the proteins they target in cancer cells; they blast those proteins and other proteins in some healthy cells. This causes side effects, some of which are so severe that doctors can’t give patients the most effective drug dosages.
Scientists and doctors know all this, of course. They’d prefer a more precise approach. They’d prefer a probe and then a ground assault.
Medicinal chemist Jian Jin is nothing like Darth Vader, but he has created a probe and sent it out into the galaxy of biomedical researchers. It’s strong, it’s nontoxic, and it’s allowing scientists to order attacks on ailments as varied as liver cancer, HIV, and cocaine addiction.
Jin, a soft-spoken scientist from China, spent ten years in the U.S. pharmaceutical industry discovering drug candidates for various targets before coming to UNC in 2008. When asked why he left Big Pharma, he smiles and pauses. “There are a lot of reasons,” he says. “Mainly I wanted the freedom to pursue the science I’m most interested in.” And that’s molecular probes.
According to biomedical researchers, for a molecule to be classified as a probe it must meet certain criteria. It has to hit its target protein without messing up other proteins. It has to be robust; it can’t work just some of the time. And the effect cannot be toxic. Drugs that are nonselective inhibitors, on the other hand, don’t have to meet such stringent criteria.
“As long as a drug is safe and effective, you don’t necessarily care how many proteins it might inhibit as long as its secondary effects are not too harmful,” Jin says. But some of those secondary effects, such as digestive problems or liver scarring, are the result of changes at the cellular level. A nonselective inhibitor can cause a cascade of cellular effects, making it harder to discern the exact influence an inhibitor has on an individual protein.
“A probe can investigate the biological function of a single protein,” Jin says. “It has to act cleanly, and only interact with the proteins it’s designed to target.” A lot of existing probes, though, are used as part of in vitro studies, but not in cells. The probes don’t necessarily have to permeate cells or be viable for use in clinical trials.
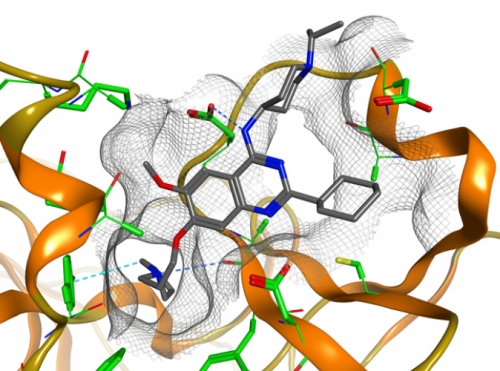
Jin’s probe, called UNC0638, is different. It targets two enzymes, proteins called G9a and GLP. They create the epigenetic code, which is full of protein and DNA modifications that determine which cells become brain cells or skin cells or lung cells. Some proteins are called writers—they create the modifications that make a liver cell a liver cell, for example. Others are called readers—they recognize those modifications. Others are called erasers—they remove the modifications. G9a and GLP are writers. But when they write too much, they cause problems. Scientists have found that both enzymes are overexpressed in many cancers, including leukemia, prostate cancer, lung cancer, and liver cancer. The two proteins have also been implicated in mental retardation, cocaine addiction, and HIV latency, the state where HIV remains in the bloodstream no matter how aggressive the treatment.
Scientists would love to know why G9a and GLP are overexpressed in these disparate conditions. That’s where Jin’s probe comes in. It suppresses only G9a and GLP so that researchers can study how the proteins affect human health.
Creating such a specific weapon took more than a year and several collaborations with scientists at UNC and elsewhere to get all the desired features into a single molecule. It started with a compound that had already been created and was commercially available for study. Unfortunately, that compound was kind of like the Death Star’s laser beam of annihilation.
In 2007, when Jin was still working in Big Pharma, other researchers published news that they had created a compound called BIX01294 that inhibited G9a. The only problem was that it was a bit toxic. How toxic? “Oh, it caused massive cell death,” Jin says. “There was little separation between the compound’s ability to suppress the proteins and its ability to destroy the cells outright.” In 2009 scientists published a three-dimensional crystal structure of the compound and GLP. Jin and colleagues in Toronto took that structure and tried to remedy its flaws through a series of chemical modifications. They wanted to change that laser beam of annihilation into a harmless but much more effective probe.
“We improved the potency of that compound, as an inhibitor, by several hundredfold,” Jin says. And those early alterations didn’t result in massive cell death. That’s because Jin’s new compounds struggled to permeate cells at all. Over the course of a year Jin’s team, including postdoc Feng Liu, kept altering the BIX compound, adding one chemical bond at a time so that the compound’s properties and abilities changed until they met all the criteria to make BIX a robust probe. “We must have made hundreds of compounds,” Jin says. Through months of trials and errors and successes, Jin’s team came up with UNC0638, a molecular probe that permeates cells and does so in a robust, nontoxic way. Jin says, “We now have a great deal of biochemical and cellular data showing that it interacts only with the proteins it was meant to.”
Jin could’ve kept his probe to himself, or at least kept it within the confines of UNC and Toronto. He could’ve patented it. “But then no one would have access to it,” he says. “And that wouldn’t increase our understanding of these targets. We made it freely available to the scientific community even before we published our findings. We want others to find new disease associations or to validate previously reported associations. Then companies can generate intellectual properties during their drug-discovery campaigns.”
Jin’s lab has given blueprints to Sigma-Aldrich, a company that sells chemical compounds to researchers, though Jin offers a cheaper route. “We’ll make it for anyone,” he says. “Now that we’ve developed two efficient ways to make UNC0638, it’s easy. We can make grams of it in less than a month.”
Mark Minden at the Ontario Cancer Institute is using Jin’s probe to investigate the roles of G9a and GLP in leukemia. Rob Bristow, also at Ontario, is using it to study prostate cancer. James Ellis at the University of Toronto is using it to study how stem cells are reprogrammed. Angelique Whitehurst at UNC is studying how the probe and proteins interact with common chemotherapies. And David Margolis, also at UNC, is using the probe to study HIV latency. Jin says that at least fifty researchers are using his probe to investigate diseases. But he’s about to lose track; most scientists get the compound from Sigma-Aldrich.
All of those researchers are preparing individual ground assaults on some of our most common diseases. They’re going to pin down G9a and GLP and find out whether these proteins are major players in the rebellion or just minor coconspirators. And if even one researcher comes up with a drug that works, maybe we’ll call him a Jedi.
Jian Jin is an associate director of medicinal chemistry at the Center for Integrative Chemical Biology and Drug Discovery at the Eshelman School of Pharmacy. Feng Liu is a postdoctoral fellow in Jin’s lab. Their main collaborator was Cheryl Arrowsmith, chief scientist at the University of Toronto’s Structural Genomics Consortium, which is committed to open access to its scientists’ and collaborators’ discoveries. Jin received funding from the National Institutes of Health and UNC’s University Cancer Research Fund. Their work was published in the August 2011 issue of Natural Chemical Biology.