Imagine a marching band. The individual musicians spread across the field in a coordinated but ever-changing motion to the music, knowing exactly when and how long to play each note. The notes meld, forming a unified series of harmonies and melodies. In a band, musicians know how to move and act by reading hand signals from the drum major. A similar thing happens in a cell. But instead of a single drum major, numerous proteins throughout the cell coordinate cell activities. Proteins send chemical signals to other proteins or cell parts to direct functions.
To send these messages, proteins in specific areas must first be activated. For a cell to move, proteins in the cell’s “feet” — extensions that attach a cell to its surface — must be turned on. But how can we understand this entire synchrony within the cell if it is invisible to the naked eye?
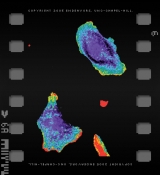
Scientists at Carolina are making it possible to visualize signaling within a cell, in real time. As they reported in the September 10, 2004, issue of Science, Klaus Hahn, professor of pharmacology, and his team of scientists — Perihan Nalbant, Louis Hodgson, Vadim Kraynov, and Alexei Toutchkine — illuminated cells using fluorescent dyes to reveal the activation of a particular protein, Cdc42. This protein, found throughout every cell of the human body, coordinates functions such as cell movement, growth, and death.
But the idea of making a cell glow isn’t new. Taking a lesson in fluorescence from a type of jellyfish that lives in the cold waters of the northern Pacific Ocean, scientists have been able to make everything from flies to rabbits glow. Scientists can attach this jellyfish protein, also known as green fluorescent protein (GFP), directly to what they want to see, lighting up the specimen of their choice. “Green jellyfish protein really blew this whole thing open,” Hahn says, because of a new technique that opened the door to using light emitting molecules as a way of studying live organisms.
But it became clear that attaching glowing molecules to a protein created problems. To study the cell this way, scientists modified the protein or added extra protein to the cell. “When you do this you can mess up and change the whole cell,” Hahn says.
So Hahn and his team developed better tools — novel dyes and innovative techniques to light up cell activity. Hahn’s group attaches the dyes to sensors that bind only to the activated form of a protein, and that light up when they bind. “This is a new way to do this kind of imaging,” Hahn says. Their method applies dye without changing the original cell or the proteins being studied, making it possible to detect the Cdc42 that occurs naturally. It is also more sensitive and responsive than previous techniques, opening the door to many important proteins present in very small amounts within the cell. “We tried to make dye structures that can sense the movement of the amino acids,” says Alexei Toutchkine, research assistant professor of pharmacology.
The rainbow of color in Hahn’s cell movies reveals a key improvement in dye techniques. Most techniques can only show the average activation of a protein throughout a large population of cells. But this isn’t how proteins really work. Activation of Cdc42 is site specific. The team’s biosensors show how much protein is being turned on at specific locations. Since Cdc42 plays a vital role in cell movement, the dye causes regions around the cell “feet” to glow red, a sign of increased activation.
Hahn says his team is a part of a wave of innovation in live-cell imaging that began at Carolina more than a decade ago. For instance, the microscopes used to see these cells have been enhanced by the work of Ken Jacobson, professor of cell and developmental biology, and Ted Salmon, professor of biology. “We work on microscopes that can take real-time movies using military cameras for night vision,” Hahn says. “We can put in tiny amounts of sensors so we don’t mess up the cell and still see what’s going on.”
These dyes hold promise in both biological research and the pharmaceutical industry, from new ways of seeing cellular behavior in action to helping create smarter drugs. “All cell activities are regulated by proteins,” Toutchkine says. “If you know how a protein behaves, you can manipulate this behavior to affect cell activity.” By ensuring that a drug affects protein activity in only a particular part of the cell, very specific drugs with decreased side effects can be produced.
The dye technology has been licensed to Genospectra, a pharmaceutical service company that specializes in analyzing cellular systems. Genospectra is developing the technology to enable scientists to create more specific drugs and examine their effects in living cells.
These new methods of real-time visualization are also changing the perception of cellular biology. Traditionally, cells were thought to act like electrical circuits — all of the parts communicating in direct, organized pathways. But Hahn and his colleagues are bringing to light the complexities behind each living cell. “We’re part of a revolution in cell biology,” Hahn says. “People are able to see the cell as a dynamic, shifting circuit where the parts are always changing and rearranging.”
Lynn Thomasson was a student who formerly contributed to Endeavors.
This research was funded by grants from Deutsche Forschungsgemeinschaft, the Arthritis Foundation, the Leukemia and Lymphoma Society, and the National Institutes of Health. The Office of Technology Development is the only UNC-Chapel Hill office authorized to execute license agreements with companies. For more information, contact OTD at (919) 966-3929 or visit research.unc.edu/otd/.