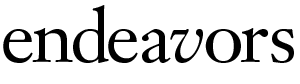Historically, scientists have worked backward in time using educated guesswork and knowledge of protein chemistry to figure out the probable evolutionary routes that proteins took to their modern form. Until recently, that is.
Matt Redinbo and his colleagues decided to start near the beginning. Redinbo and Joseph Thornton at the University of Oregon used crystallography to create the first map of the exact structure of an ancient protein. Then they virtually strolled down the evolutionary path taken by the protein more than four hundred million years ago.
Organisms typically get more complex as their proteins evolve new functions. To do that, proteins must undergo mutations that change their structure. Some of these mutations are clunky and break the protein, but others are vital and endow the protein with new traits. To identify which historical mutations were vital, Redinbo’s team compared proteins to their precursors through evolutionary time.
They reconstructed the ancestor of two hormone receptors, which function like locks in a lock-and-key mechanism. When a hormone binds a receptor, the receptor switches on a cascade of signals that allows the cell to perform a specific function. Redinbo’s ancestral protein ultimately evolved into the stress hormone receptor, or the glucocorticoid receptor (GR), and the mineralocorticoid receptor, which controls kidney functions. Humans and bony fish have both receptors; sharks, which took a different evolutionary path, have only the latter.
“This wasn’t like Jurassic Park where there was a sample drawn out of a fossil,” Redinbo says. “We statistically determined the sequence of the hormone receptor’s ancestor from an organism that lived over four hundred million years ago in the deep oceans. You can think of this creature as the last common precursor of a sea bass and a human.”
Once the team had determined the sequence of the ancient receptor, they engineered bacterial cells to produce the ancestral receptor by injecting the gene corresponding to its sequence into the bacteria. The reconstructed receptor was stable and functioned normally, which confirmed that their statistical predictions were correct.
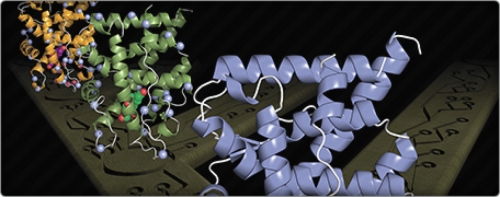
What they did next gave them unique insight into how the protein evolved. Using a biophysical technique called X-ray crystallography, they pinpointed the precise location in three-dimensional space of every single atom in the reconstructed receptor. Redinbo’s team bombarded the crystals of the ancient receptor with X rays, which bounced off the crystals. The X rays were then exposed to a film, where they formed a blueprint of all the atoms in the receptor.
The team then compared the ancestral receptor to the modern GR found in humans, and looked for mutations that changed the former into the latter. Five specific mutations stood out. These seemed to be responsible for the new function that the ancient receptor had evolved: specificity to the stress hormone cortisol.
Next the team engineered those five mutations into the ancient receptor in hopes of creating the modern GR. To their surprise, the new protein fell apart. They were either completely on the wrong track, or they were missing a vital link.
On closer examination, the team found two other mutations that happened by chance earlier in the evolutionary timescale. These two mutations didn’t seem to have any function other than slightly tweaking the fold of the protein. The new fold, though, turned out to be pivotal in strengthening the structure that the five later mutations dictated.
“It’s like cutting a window in your house and having your whole house fall down,” Redinbo says. “But if you were to alter the floor of the house just a little bit, then that new window would work out and change the way the house functions.” And that’s what they discovered: without the two silent mutations, the five specific mutations made the protein unstable.
It’s as if the ancestor were walking an evolutionary tightrope toward its new function, Redinbo says. “As random mutations kept happening, those that destabilized the ancestor would cause it to fall off and were therefore thrown out, but those that added zero value to it were not always eliminated. The two mutations that survived turned out to be essential for the five later ones that gave the ancestor a completely new function. We think that such permissive mutations may underlie the new functions taken on by most proteins in their evolutionary history.”
Evolutionary forces are intimately tied to cancer and other diseases, as well as resistance to antibiotics, says Eric Ortlund, first author of the study and a former post-doc in Redinbo’s lab. “One could certainly accelerate evolution in the lab to understand how proteins may circumvent inactivation by drugs while preserving their function,” he says.
“This wonderful piece of structural biology fills a big gap in the great conceptual puzzle of how proteins adapt to new functions,” says Ichiro Matsumura, an evolutionary biologist at Emory University. “I expect it will take time for Redinbo’s ideas to percolate through the scientific community. But that is the price one pays for thinking ahead of the curve.”
Prashant Nair is a master’s student in medical journalism at Carolina.
Matthew Redinbo is a professor in the Departments of Chemistry, Biochemistry, and Biophysics, and a member of UNC’s Lineberger Comprehensive Cancer Center. His team was funded by the National Institutes of Health, the National Science Foundation, and by a Lineberger Comprehensive Cancer Center Postdoctoral Fellowship.