As a pediatrician, Anna Spagnoli spent a lot of time monitoring kids’ heights and weights. “It’s a major sign of the health of a child,” she says. “Are they growing okay? That’s the first thing a parent usually wants to know.”
But the parents Spagnoli talked to had more than the usual concerns. They came to her because their kids were growing too slowly and breaking too many bones. Some had bone fractures that just wouldn’t heal. Many of these children suffered from osteogenesis imperfecta, a genetic disorder that makes bones fragile. Children with the disorder heal very slowly after a fracture, and may have to stay immobile during the process to prevent a rebreak.
A genetic bone disease is just one of many reasons why a patient might have trouble healing from fractures. Osteoporosis, diabetes, and other conditions also prevent normal bone formation. Of all fractures, about 20 percent don’t heal on their own. That’s six hundred thousand fractures in the United States each year.
The number seems high, but Spagnoli contrasts it with the millions of fractures that do heal normally. “This is a tissue that, 80 percent of the time, functions correctly. So if your bone isn’t repairing itself, what does it not have that other bones do?”
She and her colleagues think they know. And their research might lead to a new treatment for fracture nonunions — the broken bones that just won’t heal.
For a patient who has a fracture nonunion, the best option available today is bone graft surgery. A graft, usually taken from the patient’s pelvis, is reshaped and placed in the fracture site. But if a patient is young or has low bone density, there might not be enough healthy bone from which to take a graft. Even when a graft can be done, healing takes a long time. “One year after the graft surgery, many of these patients still have pain,” she says.
Spagnoli looks at fracture treatments through the eyes of a pediatrician. In healthy children, fractures heal well and quickly — maybe, Spagnoli says, older bones can be persuaded to act like young ones. “We want to supplement the bone’s natural healing process,” Spagnoli says.
That’s the goal of regenerative medicine, a field that looks beyond drugs and artificial transplants to produce new treatments for diseases and injuries. “Something in your own body is used to regenerate the tissue that’s not working,” Spagnoli says.
She thinks this new approach is a good one for fracture healing because bones regenerate themselves all the time. Bones aren’t static tissue: no matter what age we are, our bones are in a continuous cycle of resorbing old bone cells and forming new ones. The same process occurs during fracture healing. A web of cartilage and weak but quick-forming bone forms across a fracture site; the web is then slowly resorbed and replaced by stronger bone.
Spagnoli’s supplement for this process is a type of adult stem cell found inside bones themselves. Scientists have known about the potential of bone marrow stem cells since around 1970, shortly after the first bone marrow transplants were performed. When researchers examined the different types of cells found in the marrow, they found mesenchymal stem cells (MSCs).
“In a dish, MSCs from bone marrow turned into bone, muscle, cartilage, and other cells. What we didn’t know was whether we could get them to perform that way in a body,” Spagnoli says.
Working in pediatric endocrinology, Spagnoli had learned a lot about the hormone called insulin-like growth factor 1 (IGF-1). It’s particularly active in growing children, and it’s also known to be lower than normal in adults with osteoporosis. Spagnoli hypothesized that when MSCs treated with IGF-1 encountered a bone fracture, they would turn into cartilage cells, an early step in the fracture-healing process.
It’s a test that would have been difficult to do just five years ago. The problem, Spagnoli says, was that scientists couldn’t see what cells do and where they go once they’re inside a nonhuman animal. “Until five or six years ago, injecting cells into an animal was like putting them in a black box. You couldn’t see what the cells did until you sacrificed the animal. So you missed everything that happened in between.”
In the past few years, imaging technologies used on humans, such as CT and MRI, have become available on a smaller scale for use on animals. And scientists learned how to use the gene that codes for luciferase — the harmless enzyme that makes fireflies glow — as a tracker to watch the movement of cells inside an animal’s body.
Froilan Granero-Molto, a member of Spagnoli’s lab, extracted MSCs from the bone marrow of mice that had the luciferase gene. After treating the glowing cells with IGF-1, he injected them into mice that had fractured leg bones. The injured mice didn’t have the luciferase gene, so the injected cells stood out brightly in their blood. “You can’t see them with the naked eye, but they show up clearly in photos taken with a CCD camera,” Spagnoli says.
“This way, we could track the movement of the cells hour by hour through the mice,” Granero-Molto says. “We found that after three days, the cells got to the fracture.” They also found that the cells used CXCR4, a molecule that can home in on bone marrow, to find the fracture site. Cells that lacked CXCR4 didn’t arrive at the fracture.
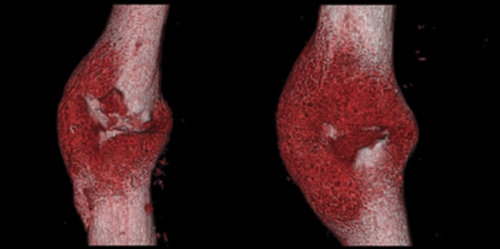
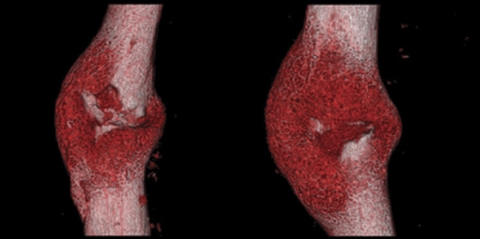
After two weeks micro-CT scans showed that mice treated with stem cells had more cartilage and bone growth at the fracture sites than did mice that didn’t receive the stem cells. The repaired bone in the treated mice was three times stronger than the repaired bone in the untreated mice. The animal model for the stem cell treatment had worked.
Success in an animal model is a crucial step toward developing a treatment for humans. But Spagnoli and her colleagues need evidence that stem cells would address a deficiency in human patients with nonunion fractures. Right now they’re planning a study of patients who have the same kind of leg fractures they studied in mice. They’ll test blood and bone marrow samples from the patients to examine their levels of stem cells, and track how well the fractures heal. They want to find out if patients whose fractures heal poorly have fewer MSCs or defective MSCs.
Spagnoli anticipates that stem-cell treatments to regenerate tissue are not far off — or at least much closer than many of the other goals of stem-cell research. “A lot of the interest in stem cells has been to treat diseases like diabetes, where there is a total degeneration of an organ — or very complex diseases like Alzheimer’s or Parkinson’s. How to cure these diseases will be a very difficult question. But how to use stem cells to help the body with a repair process it’s usually good at, such as bone healing — this is a question we may be able to answer much sooner.”
Anna Spagnoli is an associate professor of pediatrics and biomedical engineering in the School of Medicine. Froilan Granero-Molto, who presented the results of the study in June 2008 at the annual meeting of the Endocrine Society, is a postdoctoral student in pediatrics in the School of Medicine. Assistant professor of pediatrics Lara Longobardi in the School of Medicine and Vanderbilt University collaborators Michael Miga, Jared Weis, Benjamin Landis, and Lynda O’Rear were coauthors on the study. Funding came from the National Institutes of Health.