When James Raleigh developed a tool for detecting stubborn tumors, he had no idea how far it would go. It has found its way into studies of liver damage and organ transplants, and even into outer space.
Researchers use the tool, known as a hypoxia marker, to detect cells lacking oxygen. Raleigh originally intended the marker as a way to identify hypoxic cells in tumors because hypoxic cells are three times more likely to resist radiation treatment. He figured if oncologists knew beforehand which tumors were hypoxic, then they could modify radiation treatments to be more beneficial. But Raleigh and other researchers are finding out that hypoxia plays a role in many diseases and processes in the body other than cancer.
Raleigh, professor of radiation oncology and toxicology, invented his first hypoxia marker when he was a senior scientist at the Cross Cancer Institute and an adjunct professor at the University of Alberta in Canada. When he came to Carolina, one of the draws was the prospect of a collaboration with the N.C. State University (NCSU) School of Veterinary Medicine. Donald Thrall, professor of veterinary medicine at NCSU, was working with dogs that had spontaneous cancers, and he wanted to use Raleigh’s hypoxia marker to see if hypoxic cells really do resist treatment.
“The dog model is interesting because dogs grow up with people and experience the same kind of environment as humans, so when you look at dog tumors, even though dogs are a very different species, the tumors look very much like human tumors,” Raleigh says.
“It was this collaboration with NCSU that led to the development of an improved hypoxia marker that was more convenient in a clinical setting than the earlier version.”
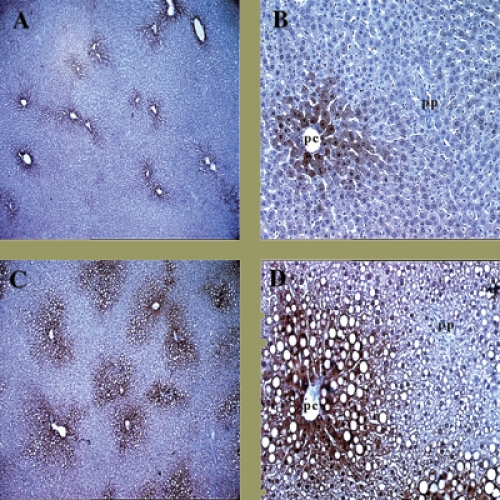
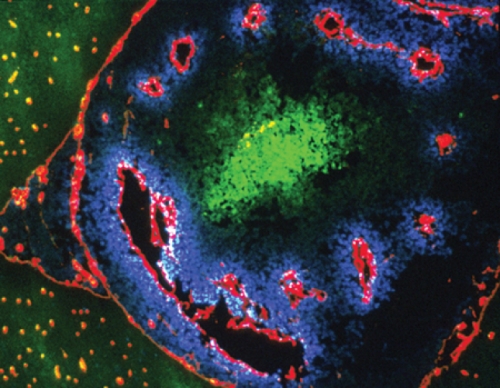
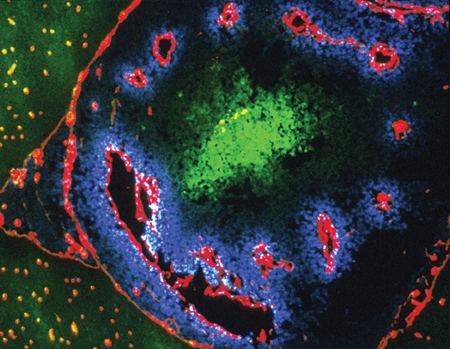
Here’s how the marker works: a drug, pimonidazole, is injected into the patient and diffuses through the whole body. Once pimonidazole gets into regions that are short of oxygen, electrons attach to it and activate the drug. The activated pimonidazole then binds to proteins in cells and is detected in biopsied tissue by special antibodies that Raleigh developed and patented and that Shu-Chuan Chou, a research analyst in Raleigh’s lab, has helped perfect. In tumors that have hypoxic cells, the antibodies appear as brown stains.
Raleigh explains that even normal tissues have gradients of oxygen. Every time you take a breath, blood vessels deliver oxygen to all tissues of the body. Cells next to blood vessels consume the oxygen, so cells that are farther away get less oxygen, causing them to become hypoxic. But hypoxic cells in themselves are not bad.
“In fact,” Raleigh says, “normal organs make use of oxygen gradients, and so as long as you don’t upset those gradients, the tissue is quite happy.”
The problem with tumor tissue is that hypoxic cells tend to resist radiation treatment, and they also tend to make tumors more aggressive by causing them to spread more quickly and invade locally—which may explain why some tumors grow back after they’ve been treated. Raleigh isn’t sure why hypoxic cells tend to be more aggressive but suspects it may have something to do with gene expression—with certain proteins found in hypoxic cells.
Figuring out which proteins are involved is proving difficult. It seems that just because a protein is expressed in tissue culture in the lab does not necessarily mean that it’s expressed within the body. This probably has something to do with the process of cell differentiation in which epithelial cells (the outer, protective cells) near blood vessels divide and move away from the vessel, slowly changing until, eventually, they flake off and are replaced by new cells. As the cells go through these changes, different proteins are expressed.
At first Raleigh thought a protein called vascular endothelial growth factor (VEGF) was involved in making hypoxic tumors aggressive because, according to tissue culture experiments, hypoxic cells produce this protein. VEGF can stimulate blood vessel growth and, in theory, create faster growing tumors. But by using the hypoxia marker on actual biopsied tumor tissue, Raleigh and Mahesh Varia, professor of radiation oncology, found that the majority of hypoxic cells in human tumors did not produce VEGF
“This was a big surprise to us and has led to a whole new way of thinking about what’s going on in tumors,” Raleigh says. “Ultimately, I think differentiation in hypoxic cells is going to give us a clue as to why hypoxic cells are more aggressive.”
In the meantime, Raleigh’s marker is proving useful in other labs across and beyond campus. Ron Thurman, who was a professor of pharmacology (he died on July 14, while this issue was in press), used the marker to test alcohol-associated liver damage. In an interview in May, Thurman explained that when you drink alcohol, your liver becomes more hypoxic—the gradients of oxygen become steeper because alcohol stimulates oxygen consumption in the cells near blood vessels, making less oxygen available to the rest of the tissue. “This in itself is not a problem,” Thurman said, “but if you drink alcohol continuously, it causes the cells to consume more oxygen, so that those last cells don’t get any oxygen at all.”
Gavin Arteel, who was a graduate student in Thurman’s lab and is now a research assistant professor of pharmacology, used the hypoxia marker for his dissertation research to see if it was hypoxia that was causing liver damage. By continuously feeding rats alcohol through a tube in their stomachs for weeks, Arteel found that the first phase of damage, known as steatosis and characterized by the formation of fat globules around the vein, is accompanied by an increase in hypoxia. It seems that the presence of hypoxia generates free radicals that contribute to fatty livers and, ultimately, fibrosis and cirrhosis.
“What’s great about Raleigh’s marker in these experiments,” Thurman said, “is that you can take an awake animal with no anesthesia and find out what’s really happening under physiological conditions. Before the marker, we had to cut the livers out of the mice and supply oxygen with a pump. Now we can inject pimonidazole directly into the mice and biopsy a small piece of tissue from the liver, which isn’t painful for the mice.”
Thurmans’s lab is also using the hypoxia marker in a study with cyclosporin, an immunosuppressive drug used in transplant patients to prevent the rejection of donated organs. The downside to cyclosporin is that it can cause severe kidney damage. So Thurman’s idea was to use the hypoxia marker to see if cyclosporin was increasing hypoxia in the kidneys. Studies indicate that it does. Now the goal is to come up with an alternative drug or figure out some way to counteract the cyclosporin, so it doesn’t increase hypoxia.
A little farther away from Carolina, Ted Gross, associate professor of orthopaedics and sports medicine at the University of Washington in Seattle, is using the marker to study bone loading—changes in the bone—that occurs because of weightlessness, for example, when astronauts are in outer space for extended periods of time. He thinks it might have something to do with hypoxia in the bone. While he hasn’t confirmed the theory, he has done experiments on turkeys in which he clamps the bones in their wings together to manipulate bone loading. After simulating weightlessness in the bones, he measures the amount of hypoxia in the bone tissue. He’s not sure of the exact mechanism behind the effect of loading on bone structure change, but he’s found that one of the early events is the generation of hypoxia in the bone tissue.
While Raleigh isn’t directly involved in all of the research projects using his marker, he says it’s exciting to know that he’s developed a tool that is useful to a lot of different people. “People around the world are using it in ways I hadn’t even conceived,” he says.
Catherine House was formerly a staff contributor for Endeavors.
Raleigh has two patents on his hypoxia marker, so users must get permission from Natural Pharmacia International, which produces the product. For information on reporting inventions, contact the Office of Technology Development.
Editor’s note: Thurman died June 14, 2001, of a heart attack. He leaves a long, distinguished record of research, only a small portion of which is mentioned here.