In a tunnel two thousand feet below the desert near Roswell, New Mexico, a lone beam of light from Jack Griffith’s headlamp struck a thick wall of ancient salt. Griffith had no idea what was trapped inside this 250-million-year-old crystallized formation. Ancient fossils? Bacteria? Nothing? He didn’t dream of finding an organic molecule that might help scientists find life on other planets. That sort of idea usually belongs above ground in Roswell, de facto home of ufology and alien conspiracy theories. But Griffith’s science project turned out to be almost as surreal as Roswell, and a whole lot more provable than a UFO.
Jack Griffith isn’t known to traipse into the wild searching for odd and ancient matter. He is, though, something of a legend for his work photographing the tiniest of things with his electron microscope. He figured out a way to see the finer details of DNA, and he took the first photo of DNA bound to a known protein. Such work has helped biochemists analyze macro-molecules of all shapes and sizes. (See “Seeing Things Jack’s Way,” Endeavors, Fall 2004.)
One such biochemist is Bonnie Baxter. Several years ago, she asked Griffith to photograph bacteria she had found in the Great Salt Lake. He agreed, and while peering at Baxter’s samples he saw surprisingly large amounts of bacterial viruses in the background.
“We didn’t expect to see that,” Griffith says. “Scientists had seen it in other salt environments but never in the Great Salt Lake. The viruses looked like ones that grow in people.”
Curious but focused on his main research, Griffith had two high-school interns spend the summer studying the bacterial viruses. But Griffith’s curiosity eventually got the best of him. He knew that really old halite formations exist around the world; what if bacteria and their viruses were trapped inside these ancient salt crystals?
Baxter sent Griffith salt crystals from an old mine in Utah, but he couldn’t find many with inclusions, the pockets of water that might contain very old organic material. Geologists told him that surface water had continually leaked into that salt formation, redissolving the salt over and over and casting doubt on the age of anything encased in the crystals.
“That salt deposit was geologically trashed,” Griffith says. “So then it became a matter of, well, should we drop this, or should we get a little more serious?”
Griffith read up on ancient halite formations and found out that the most promising, undisturbed salt deposit lies two thousand feet below the desert thirty miles southwest of Roswell.
Last summer, Griffith flew to El Paso, Texas, and drove two hundred miles into the middle of the desert until he came upon an inconspicuous mining operation.
“Unless you knew what this was you’d drive right past,” he says. “There are no signs. It has a very low profile, yes, except for all the security.”
Turns out, this is no salt mine. It’s a dump for nuclear waste. Or, as the U.S. Department of Energy (DOE) calls it, a Waste Isolation Pilot Plant. Either way, it’s where the DOE buries transuranium waste from old nuclear warheads.
But Griffith says this is no pilot plant.
“There’s almost an entire city cut into that gigantic salt deposit a half-mile underground.”
In the 1990s, the DOE dug a mine shaft through 2,000 feet of rock to reach the Permian Salado Formation, a 250-million-year-old conglomeration of halite — crystallized sodium chloride, also known as salt. The Salado is 2,000 feet thick and extends for miles underground. Using a gigantic drill, the DOE has hollowed out miles of tunnels and dozens of rooms as big as football fields to store thousands of barrels full of nuclear waste. The 100-gallon barrels are stacked three high, wrapped tight, and then sealed behind a twelve-foot cement wall. Geologists say that over time, the salt will act like a glacier, slowly covering the barrels and encasing them permanently.
Griffith says that most geologists who have studied the Salado are confident the formation is too deep under rock to have ever been penetrated by surface water. This means that the Salado very likely had remained unperturbed for 250 million years, since the continents were clumped together in one landmass called Pangaea. When the continents began drifting apart, a large pool of oceanic salt water was trapped inland near the equator. The water eventually evaporated, leaving an enormous salt deposit that crystallized and was covered by sedimentary rock. That part of Pangaea is now southeastern New Mexico.
Most macromolecules are thought to degrade well before 250 million years have passed. DNA definitely isn’t supposed to last that long. But few people have looked for it in such a strange place.
Griffith pulled up to the outer fence at the nuclear waste site, watching a conveyor belt dump tons of large salt crystals onto enormous trucks. There, he met geologist Dennis Powers, a DOE consultant and Salado formation expert who handed Griffith a hard hat with a headlamp. They stepped inside an elevator and whooshed down through two thousand feet of darkness.
“Whether you’d like this depends on whether you’re claustrophobic and like insects or not,” Griffith says. The elevator let them off near the intake shaft that sucks in air — and bugs — from the surface.
“There’s a colony of black widow spiders, thousands of them, just hanging out by the intake shaft waiting for insects to be blown into their nests,” Griffith says. “We did our sampling elsewhere.”
Powers and Griffith hopped on an electric-powered cart and navigated their way through the eerie caverns, passing the many rooms full of nuclear waste until they came upon a freshly cut wall of halite that glowed when lit. There they found a perfect chunk of salt that Powers chipped away at, searching for inclusions. He found a lot.
For two days Griffith and Powers, along with Bonnie Baxter and DOE physicist Roger Nelson, collected chunks of halite and stuffed them into Ziploc bags. All told they hauled out more than one hundred pounds of salt, packed the bags into black fiberglass camera cases, and FedExed the lot to Chapel Hill.
Back at his lab, Griffith and graduate student Smaranda Willcox searched for inclusions. They sterilized the surfaces of the crystals to kill any bacteria that might have contaminated them since they were removed from the Salado. Then, while peering through a regular microscope, Griffith and Willcox clamped a crystal to a drill press and, with a very fine needle, drilled into the salt until they reached the trapped water. Then they used a glass microcapillary to remove the salt-saturated water.
“This was a complete nuisance,” Griffith says. “And we didn’t get much material.”
They did this eighty times before Griffith found a way around. He whittled the crystals to their pristine cores before dissolving them in ultraclean water.
Using the bulk-dissolved crystals and the salt water recovered from the tiny inclusions, Griffith and Willcox prepared the samples for viewing in the simplest way possible, in order to avoid contamination. They applied a drop of the sample to a three-millimeter round copper screen coated with carbon, washed it with water, air dried it in a vacuum, and finally coated the sample with tungsten so that the microscope would contrast whatever was in the sample with the background.
Then Griffith and Willcox popped samples into the electron microscope and searched for the remnants of ancient life. It didn’t take long to find something. And at first they weren’t sure what it was, because they had never seen anything like it. Griffith searched through dozens of samples and saw this same strange substance. Then he found another substance he didn’t expect. It was DNA. And it had to be 250 million years old.
But other scientists had found supposedly ancient organic matter, only to face questions about its true age. Is the DNA Griffith found truly ancient, or is it a modern contaminant of ancient samples?
Ever since scientists figured out how to study the tiniest of things, they’ve been trying to find remnants of the oldest life forms on Earth. They’ve dug up skull fragments of bears and Neanderthals from 100,000 years ago. They’ve tracked down dormant bacteria in ice glaciers that date back 750,000 years. And they’ve unearthed 11-million-year-old cellulose — the chief component of a plant’s cell wall — in Canada’s arctic forest.
Griffith says this work is pretty much accepted as fact within the scientific community. Other findings, though, are kind of murky. In the 1990s, for example, researchers found bacteria in amber — fossilized sap — that was between twenty-five million and forty-five million years old. “That’s the Jurassic Park stuff,” Griffith says.
But Griffith and others say that such amber might not be the best stuff for this kind of test. The bacteria inside might not be very old. Also, scientists didn’t find a colony of bacteria to study under a microscope. They put a sample from amber on a Petri dish coated with nutrients and hoped that this new environment would be conducive for a dormant bacterium to multiply.
“You do this a couple hundred times and see what happens,” Griffith says. The colony is definitely not ancient, he says. It might have grown from something ancient. Or maybe not.
“This bacterium could’ve been something that floated in from your hair or anything else,” he says. “You just don’t know.”
Last year, NC State researchers reported finding fragments of proteins from dinosaur eggshells dating back sixty-eight million years. Griffith says their findings are certainly valid, but to detect ancient DNA, researchers normally rely on the polymerase chain reaction, or PCR, to amplify their samples. And PCR, like growing colonies on a plate, is an amplification method.
“You do a PCR and out comes a test tube full of DNA,” Griffith says. “But only one DNA molecule is needed to start that reaction. Does the PCR truly amplify something that was an ancient molecule, or something that was stuck to the tube?”
Griffith says PCR tests have a bad habit of amplifying present-day contaminants. The only thing an electron microscope amplifies is an image. Still, contamination could play a role.
Griffith did see DNA trapped inside ancient halite. And he thinks it’s the remains of a 250-million-year-old organism, which would make it the oldest macromolecule ever found.
“This flies in the face of biochemical experiments that show that DNA probably shouldn’t last that long,” Griffith says. “But the high-salt environment of the Salado is probably quite protective to DNA.”
He wrote his paper and sent it to journals, but the biochemists who reviewed it were skeptical because Griffith found only a trace amount of DNA.
“When you see things only occasionally with an electron microscope, you worry,” Griffith says.
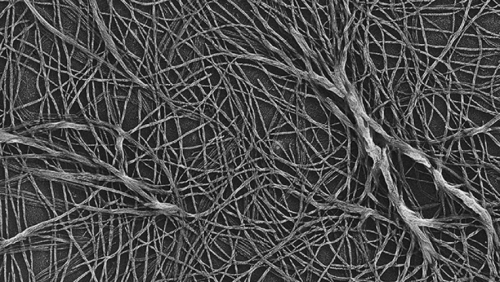
His lab is now using biochemical assays to confirm or disprove his findings. But he’s confident he found ancient DNA, and one reason is that he found so much of that other strange substance. It looked a lot like cellulose, only no type he had ever seen.
“Modern cellulose is clumped together,” he says. “This ancient stuff was untangled. We were tripping over it, there was so much.”
Biologist Ann Matthysse, also at UNC, helped Griffith figure out if it was really cellulose.
Matthysse told Griffith to douse the ancient material in a mixture of sodium hydroxide and sodium borohydride at sixty-five degrees Celsius. This stuff will eat through leather, disintegrate insects, and burn away dead skin. But it won’t harm cellulose, and nothing happened to Griffith’s sample. Certain enzymes, Matthysse told him, will chew up a lot of things, but they won’t harm cellulose. These enzymes didn’t harm Griffith’s sample either.
Then Matthysse told Griffith that a protein enzyme called cellulase chops up cellulose so it can be degraded. And that’s what happened to Griffith’s substance.
He snapped pictures with the high-resolution digital camera inside his electron microscope, and then he remembered that Malcolm Brown, a former UNC biologist now at the University of Texas, had taken some of the only photos of cellulose microfibers with an electron microscope.
“His photos looked very similar to ours,” Griffith says.
It’s cellulose. Griffith has no doubt. And it’s at least 250 million years old, by far the oldest native macromolecule ever found. Griffith says that analyzing this cellulose may reveal more details about Earth’s ancient biosphere.
It might even give us some clues about places other than Earth.
When Griffith sent his findings to journal editors, they gave the paper to geologists. The geologists not only questioned the likelihood of finding DNA in halite, but a few of them also wondered if the Permian Salado Formation is really that old and undisturbed.
Griffith rewrote the paper, adding nearly everything known about the Salado so that the geologists would be satisfied.
He also removed his findings about DNA and focused on proving the cellulose side of the story. The presence of ancient cellulose in Griffith’s samples led him to an interesting conclusion: when scientists go seeking life on other planets, cellulose microfibers — not DNA — may be the best thing to look for.
He sent his paper to the journal Astrobiology, which published it in April 2008.
Griffith’s theory, which he admits is a bit philosophical, is that life on other planets would likely be carbon-based. The six-carbon glucose molecule is the fundamental energy currency of most known carbon-based life forms, including the most primitive bacteria that existed 1.6 billion years ago, before there was an oxygen-rich atmosphere. Cyanobacteria are the modern-day descendents of those ancient primitive bacteria. And cyanobacteria create cellulose out of glucose molecules.
“It’s very likely that any of the earliest life forms on other planets would learn how to stick these glucose units together to make this semi-crystalline five-nanometer cellulose microfiber,” he says. “It’s stiff and rigid, and very few things will break it down.”
When cells die, cellulase enzymes typically are not around to break up cellulose. That means that cellulose won’t degrade quickly, as Griffith’s findings prove. And even if cellulase is present, it cannot completely digest the thirty-six-glycan chain that makes up a cellulose microfiber. DNA and proteins, on the other hand, rapidly degrade when enzymes are released after cells die.
Cellulose microfibers exist in a semi-dehydrated state. This means that cellulose might cope better than other macromolecules with the dry conditions found on other planets in our solar system. Cellulose would probably also withstand radiation levels like those on Mars better than other macromolecules would. Scientists already suspect that Mars and other planets have evaporites — mineral sediments created when surface water evaporates. Griffith says that scientists should explore halite in these evaporites.
“Who knows?” he says. “Cellulose might just be the electron microscoper’s version of little green men.”
Jack Griffith is the Kenan Distinguished Professor of Microbiology and Immunology in the School of Medicine and a professor of biochemistry. Ann Matthysse is a professor of biology in the College of Arts and Sciences.
Griffith used a stipend from his Kenan Distinguished Chair award to fund this research. He is now seeking a grant from the National Science Foundation to research older salt deposits around the world; there’s a 400-million-year-old halite formation thousands of feet below Detroit.