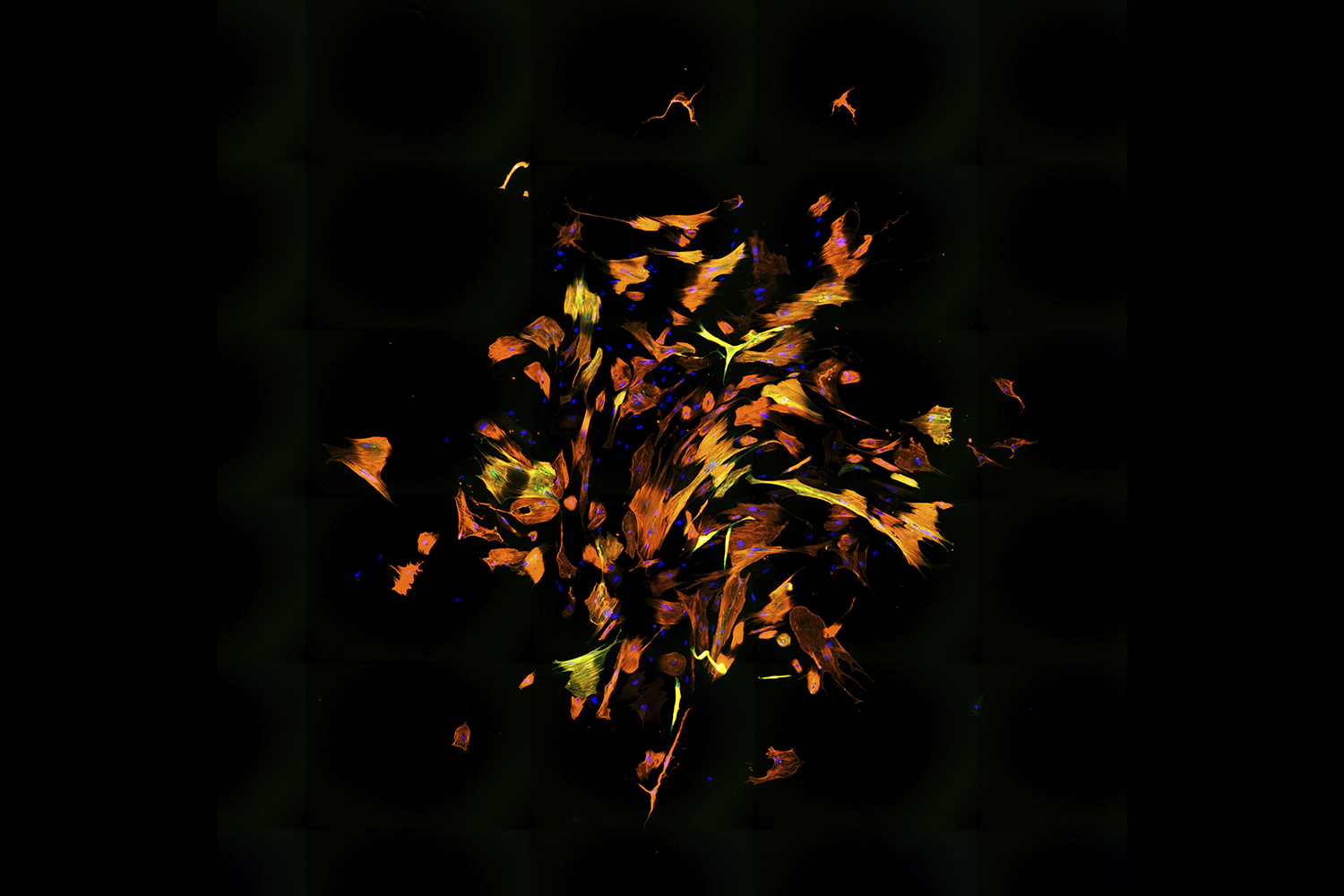
A swirl of oranges, yellows, and reds propels outward like a flurry of leaves on a windy autumn day. The image looks like an abstract painting, its bright, sporadic strokes in stark contrast to the black void that is the background.
This is not a canvas in an art gallery. It’s an image from a microscope. In fact, it’s dozens of images that have been stitched together by UNC-Chapel Hill PhD student Benjamin Keepers, who works in the lab of Li Qian. A heart researcher within the McAllister Heart Institute, Qian focuses on transforming damaged, “non-beating” scar tissue cells into “beating” muscle cells.
This image represents a culture of human fibroblasts — cells that form our connective tissue and aid in the healing of wounds — that have been reprogrammed into cardiomyocyte-like cells, which are cells that help the heart contract. The colors come from staining: DNA is blue and two different cardiomyocyte markers are orange and green.
Qian is one of many researchers at Carolina creating gorgeous images to study biological processes, address human disease, and propel science forward.
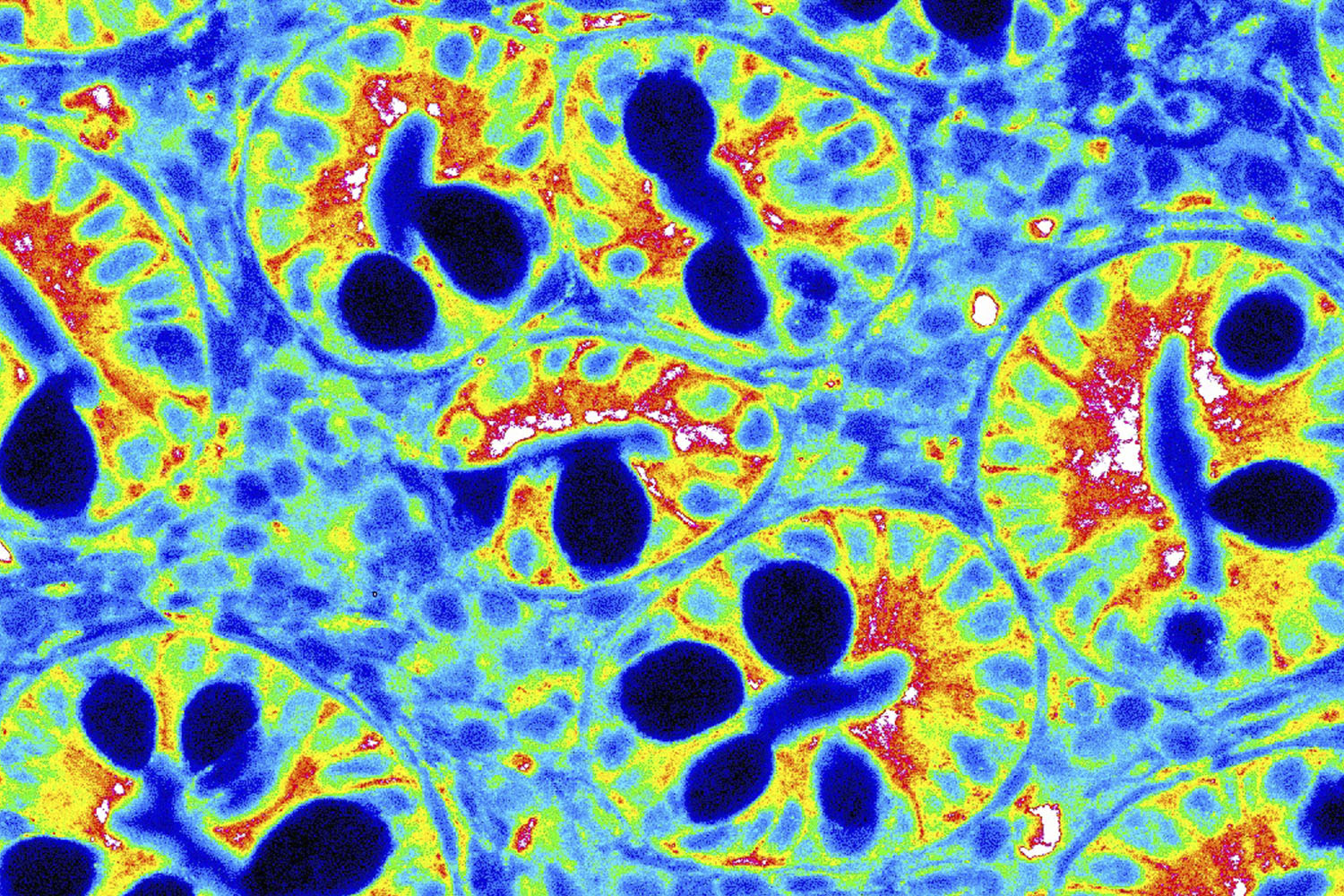
These psychedelic smiley faces come from an area of the body that we definitely want to keep happy: the gut. More specifically, they are cells within the duodenum, the first part of the small intestine, and have been expressed using a heatmap. Until recently, these cells were thought to be part of a passive, nutrient-absorbing barrier. But scientists have discovered that they elicit an immune response when needed. Christina Graves, a postdoctoral researcher in the Adams School of Dentistry, studies these cells to understand how environmental and psychological stressors rewire the neuroimmune system and affect mucosal surfaces, like the gut and oral cavity.
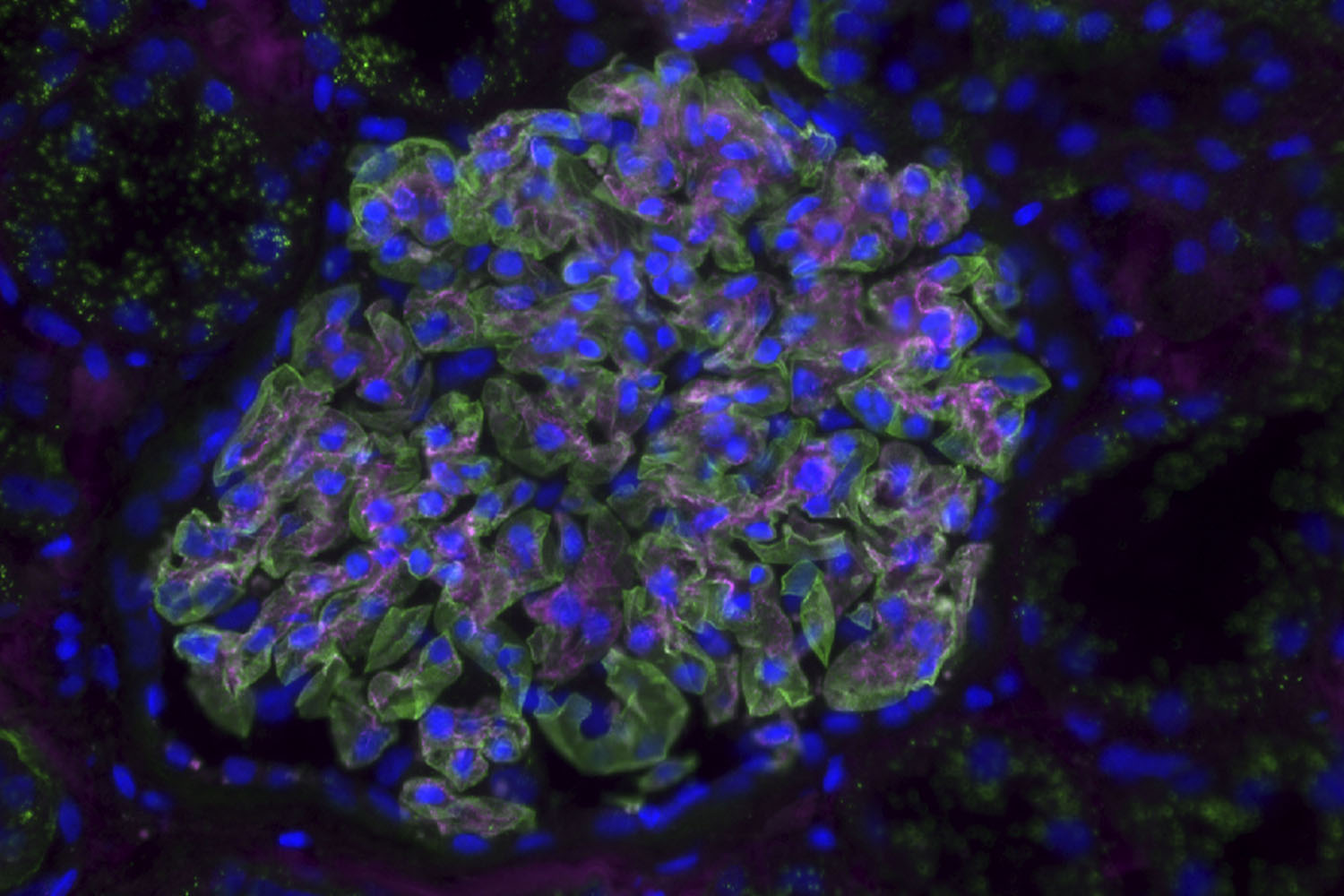
Soft greens, blues, and purples come together in this microscope image to showcase the beauty of the glomerulus, the blood-filtering unit of the kidney. These cells were acquired from a donor more than 90 years old. Cell biologist and physiologist Lori O’Brien collects these samples to study the changes that occur with kidney aging and disease to improve treatments.
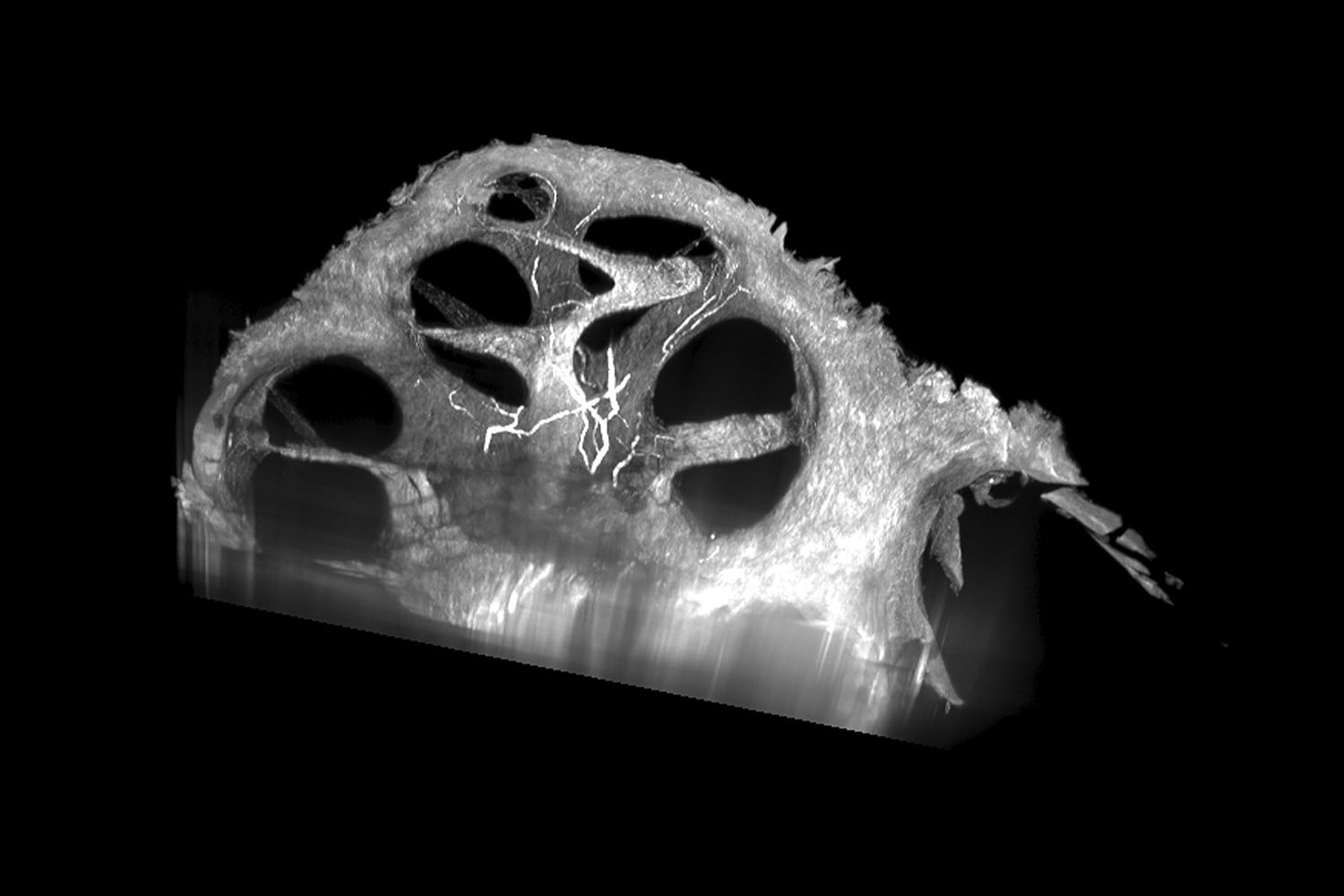
Although embedded in bone, the human cochlea is actually a spiral cavity found within the inner ear that translates sound to the brain — which processes it and allows us to hear. Within the Department of Otolaryngology, researcher Kendall Hutson uses software that can digitally slice the cochlea along any plane so that he can study it in detail. Understanding the roles of different cell types within the cochlea will help improve the next generation of cochlear implants.
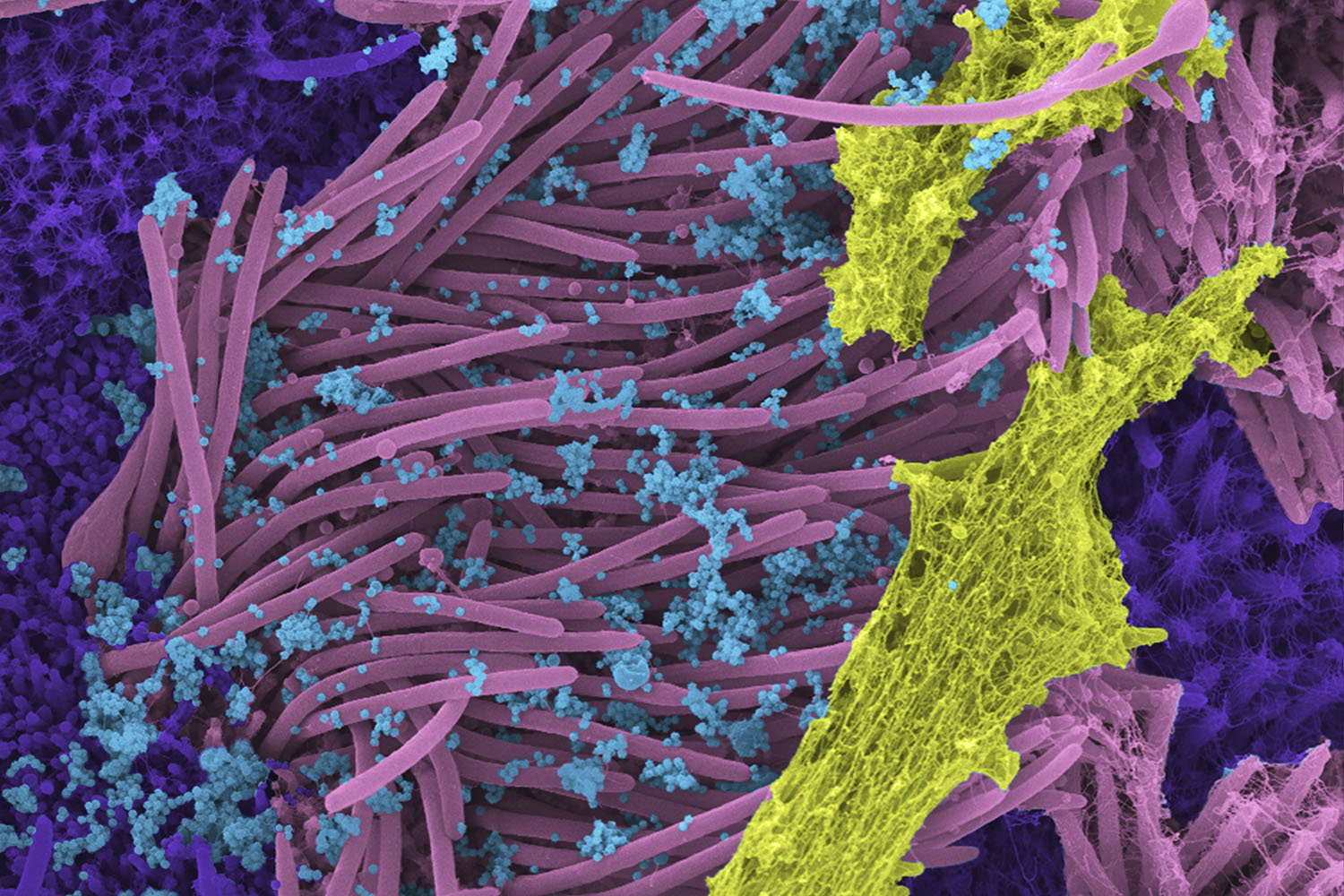
This scanning electron microscope image was colored in post-processing for the New England Journal of Medicine. It shows human airway cells infected with SARS-CoV-2. Blue viruses target and hijack cells with cilia — the pink hair-like projections that move microbes and debris up and out of the airway — covered in yellow mucous and surrounded by purple secretory cells. Created by Marsico Lung Institute researcher Camille Ehre, this image has been shown around the world to illustrate the intensity of viral shedding from the airways and was recently used in a yet-to-be-released study in collaboration with coronavirus expert Ralph Baric to improve our understanding of how mucus and inflammatory markers can affect viral entry, replication, and transmission.
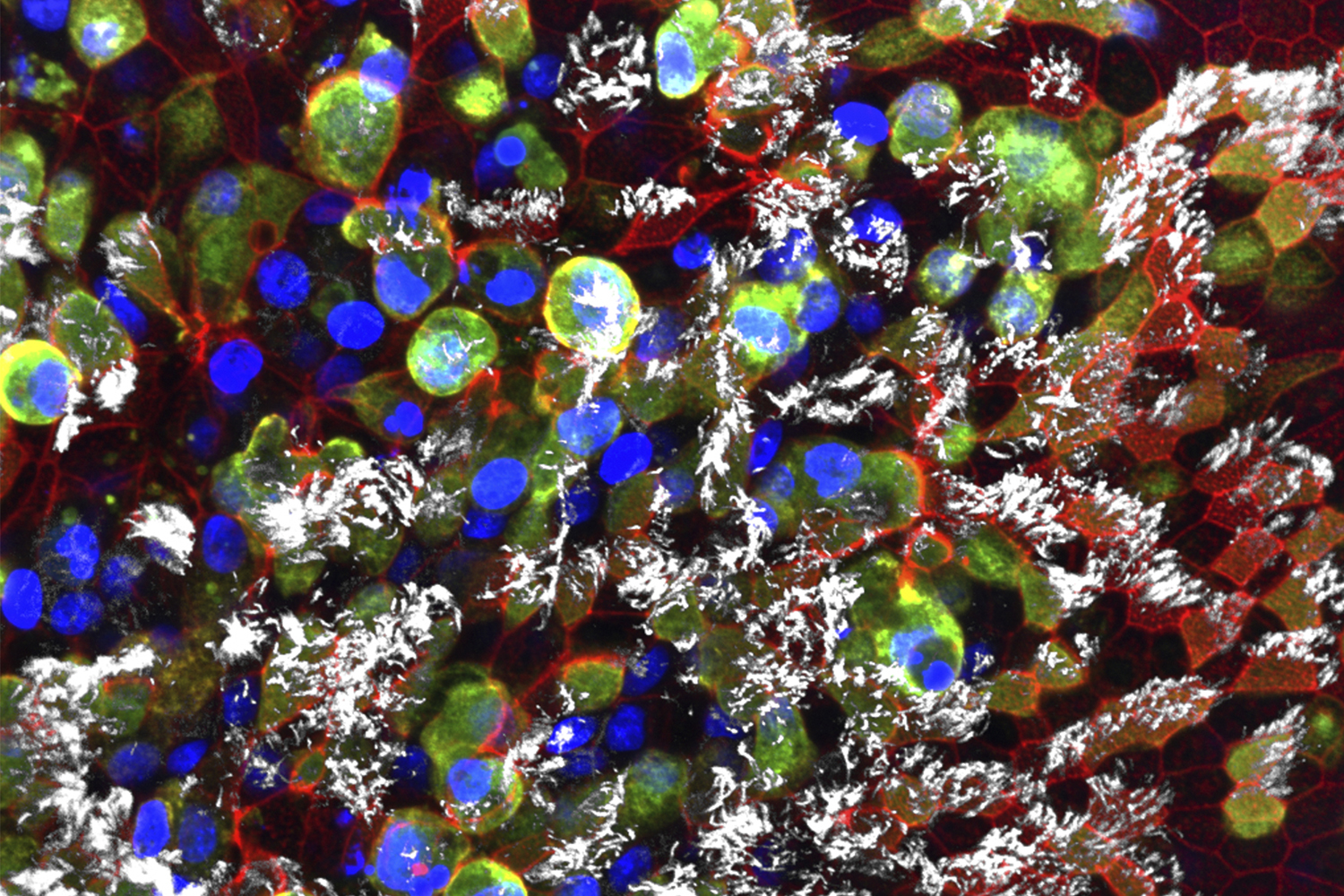
Scott Randell, another researcher within the Marsico Lung Institute, also studies how the novel coronavirus impacts the lungs. This image, taken by PhD student Rhianna Lee, shows the same event as Ehre’s — with green- and gold-colored viruses infecting white ciliated cells, blue nuclei, and a red protein called actin — but it was depicted using a confocal microscope, which creates an extended focus view of a 3D stack of images. Randell and researchers in his lab study many respiratory tract diseases, with a particular focus on cystic fibrosis. They have studied other coronaviruses for over a decade and ramped up during the pandemic to research SARS-CoV-2.
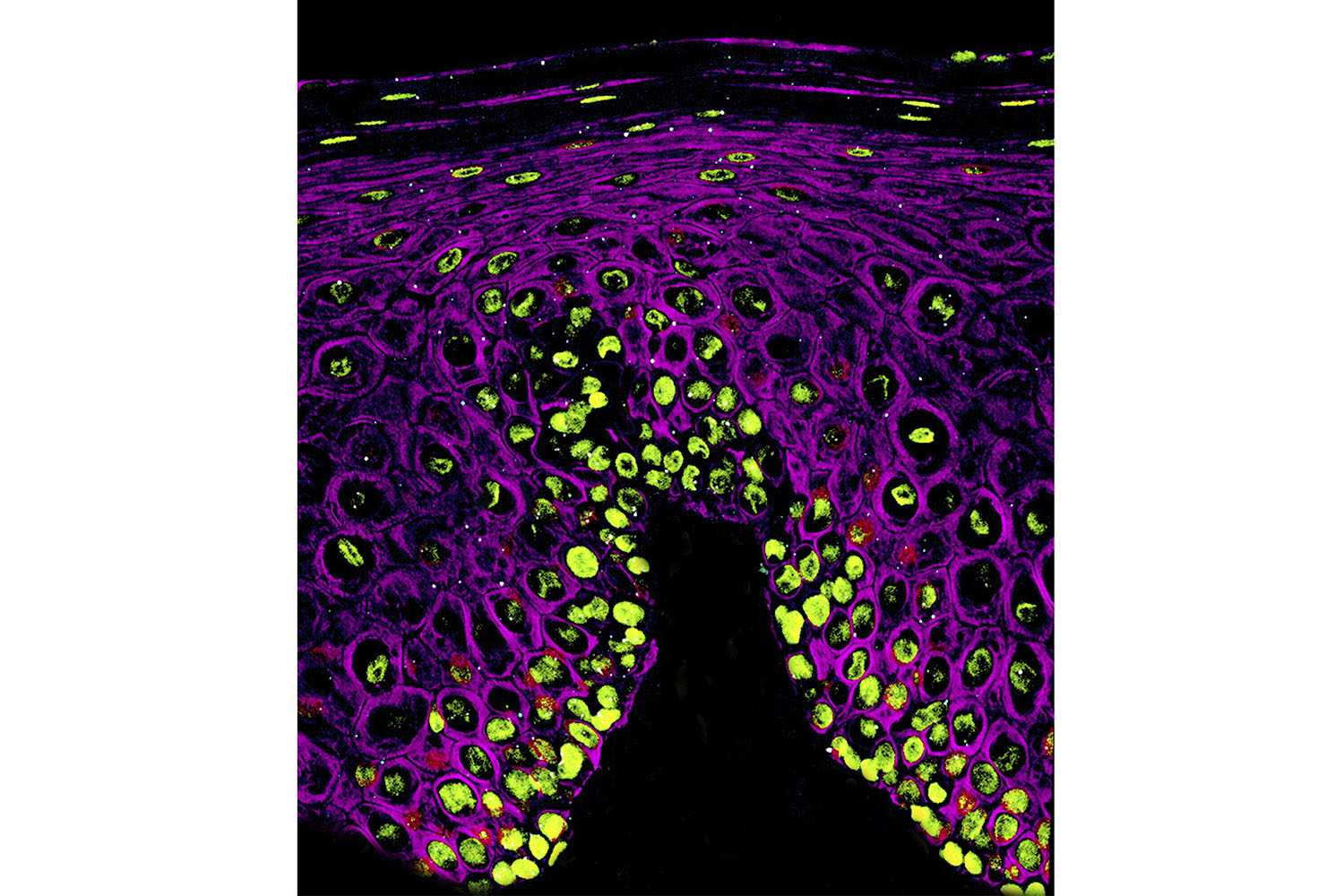
The mouth is another primary site where COVID-19 infections begin. Here, teeny-tiny white viruses infiltrate the cells that line the oral cavity. The green blobs are nuclei and the mesh-like purple structures are a protein called cytokeratin. Kevin Matthew Byrd — a professor in the Adams School of Dentistry who studies how the lining of the oral cavity protects the head and neck from microbes, toxins, and injury — created this image in collaboration with the lab of Blake Warner at the National Institute of Dental and Craniofacial Research. It was the first image to show this infection in the mouth and was published in Nature in March 2021.
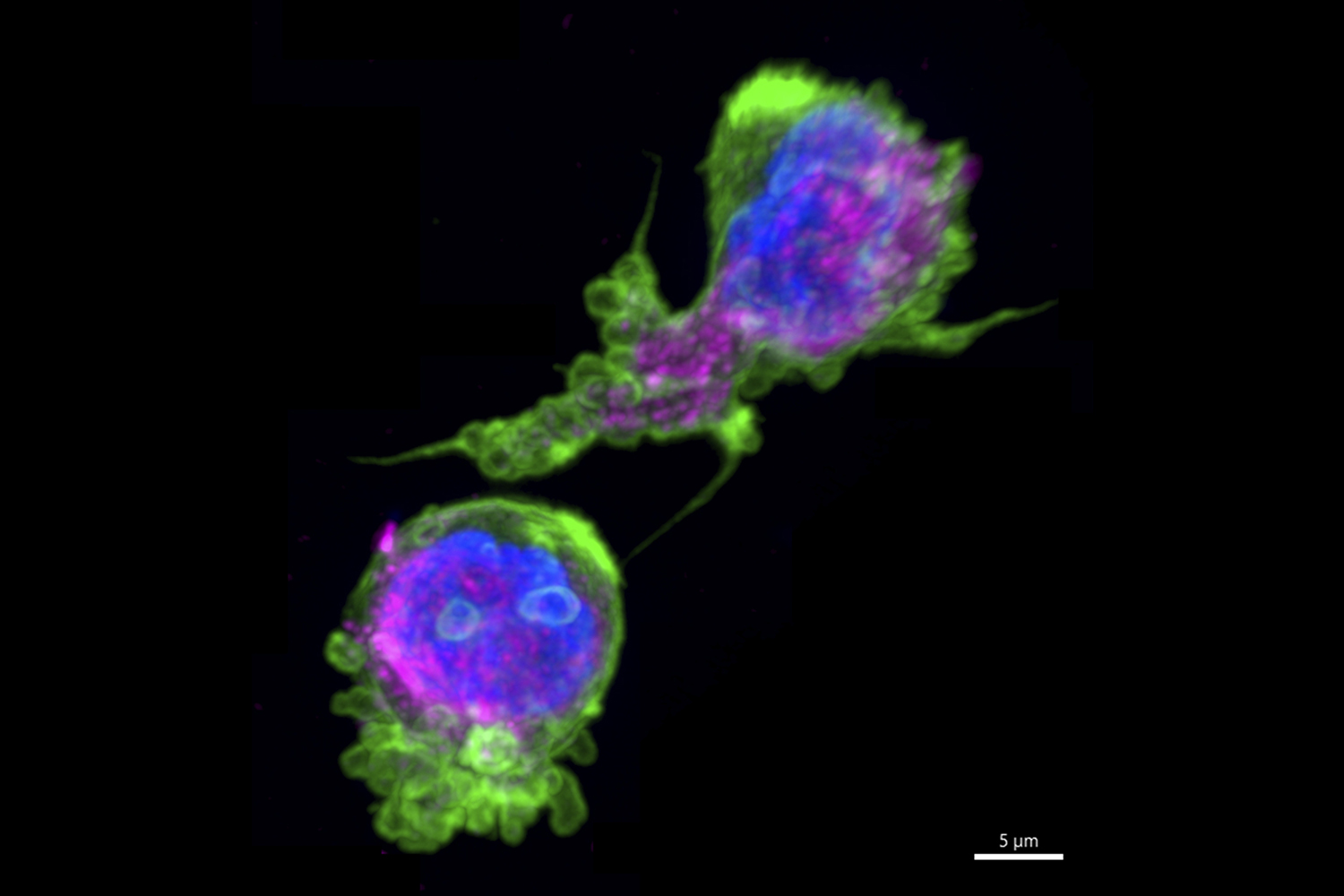
Biophysicist and cell biologist Maryna Kapustina captured these frolicking white blood cells during their migration in an environment mimicking biological tissue. They are comprised of magenta microtubules and a green protein called actin — both cytoskeletal structures that provide the mechanical support for functions like division and movement. Studying the changes in the cytoskeleton during cell movement informs how cells transform to successfully navigate inside an organism. The results of this study are important for developing novel therapeutic strategies for treating diseases, especially for preventing cancer cell migration during metastasis and for tissue design in regenerative medicine and bioengineering.
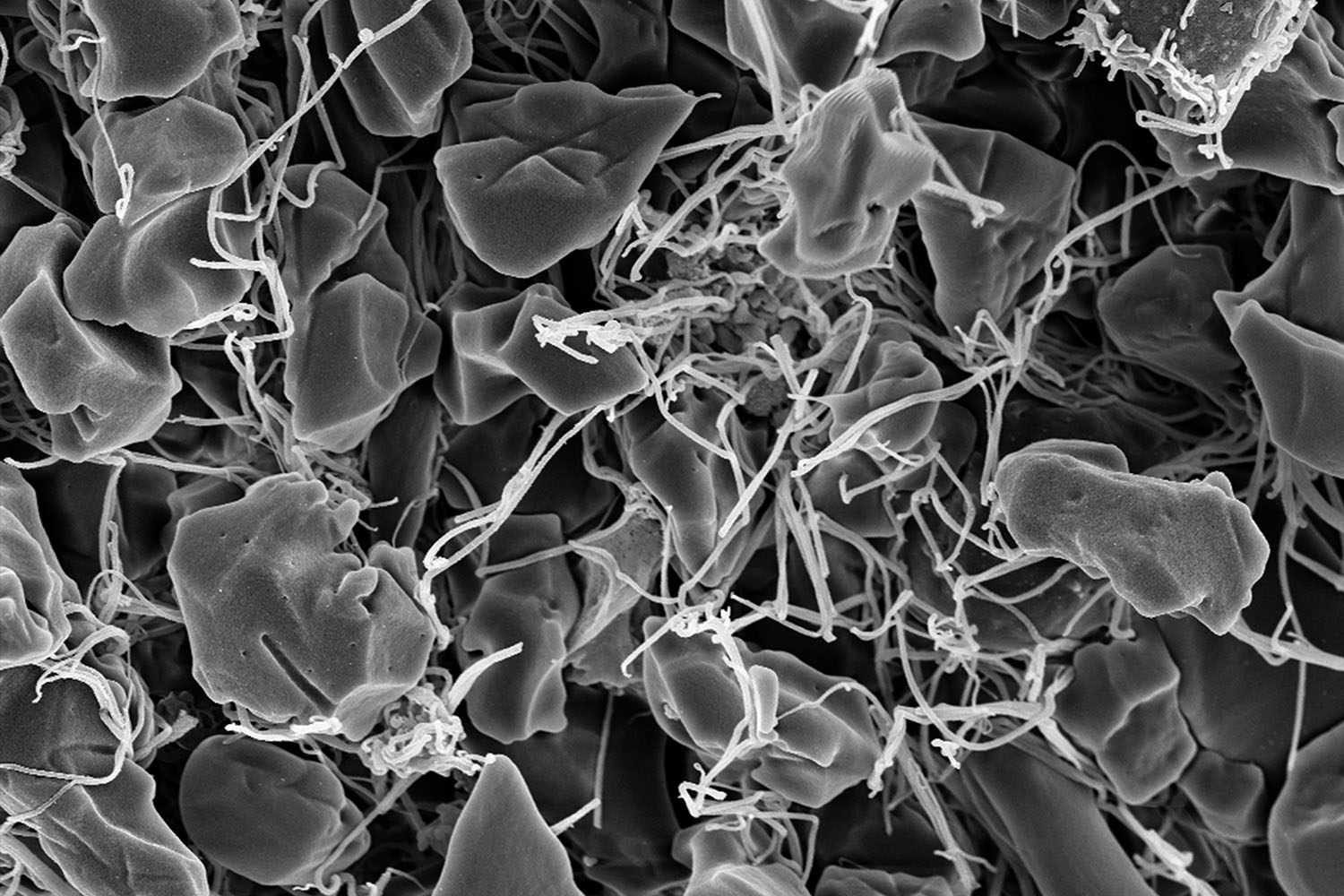
These blood cells, which are surrounded by a stringy protein called fibrin, are misshapen due to sickle cell disease, an inherited red blood cell disorder that prevents oxygen from being carried throughout the body. For this project, Erica Sparkenbaugh, Rafal Pawlinski, and Nigel Key — investigators in the UNC Blood Research Center — compared blood clot formation in patients with sickle cell disease to similar processes in healthy adults.
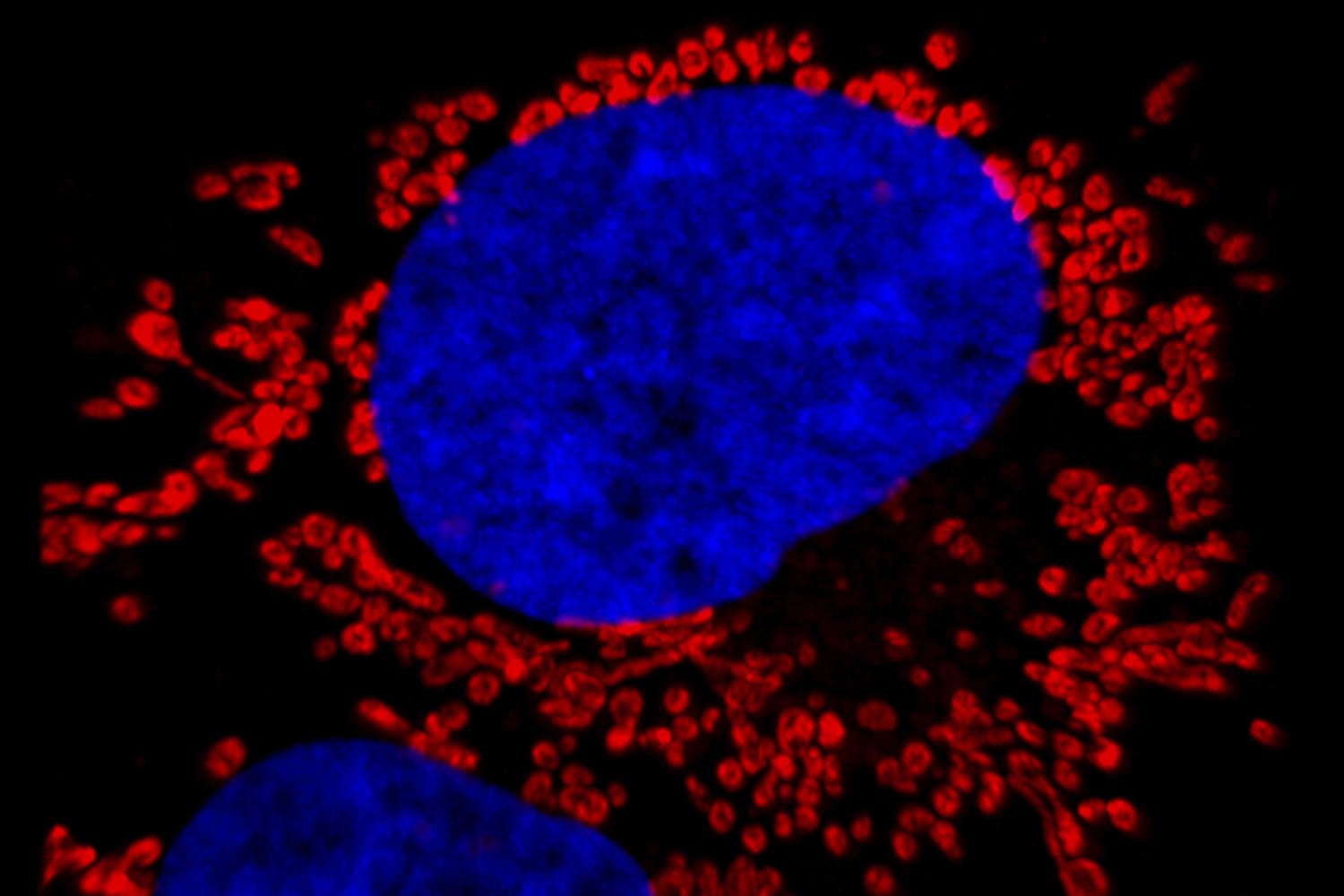
Captured by Maryna Kapustina and You Li, this human liver cancer cell shows a blue nucleus surrounded by small, red organelles called mitochondria, which provide the cell with the energy needed for survival. Infectious disease researcher Stanley Lemon and his lab culture these cells — which support infection with the viruses that cause hepatitis and liver cancer — to improve their understanding of the interplay between the virus and its host cell, the outcome of infection, and the risk of disease.
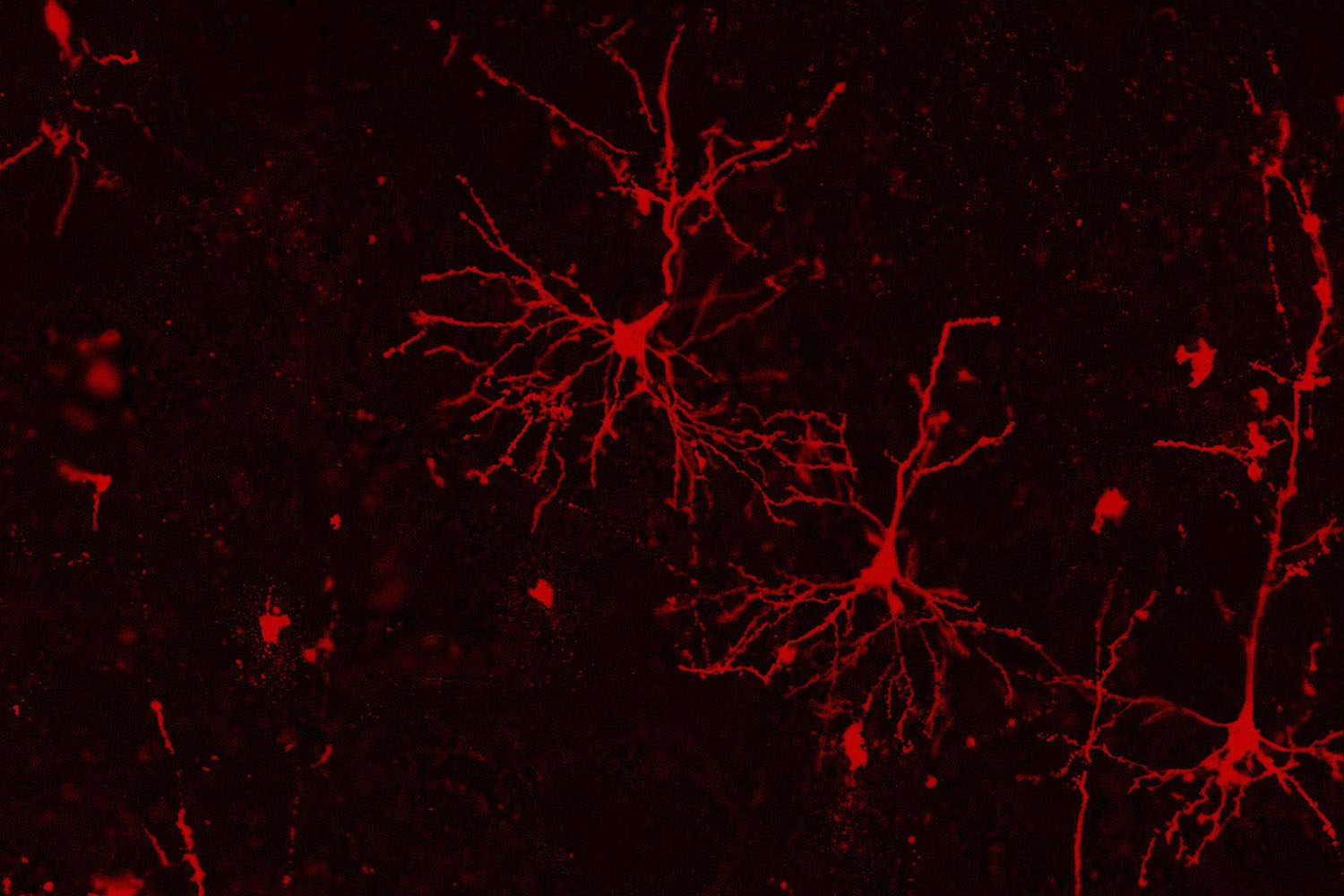
Madison Rose Glass, a graduate student within the lab of neuroscientist Jason Stein, uses a technique called Golgi staining to highlight cortical neurons in post-mortem human brains. Stein and his researchers study how changes in our genome affect our brains, which could lead to neuropsychiatric illness. These images from deceased human brains will be used to count differences in synaptic spines — the connections that a neuron has to other neurons — from people of different genotypes to determine if certain genotypes lead to more or less neuronal connections.
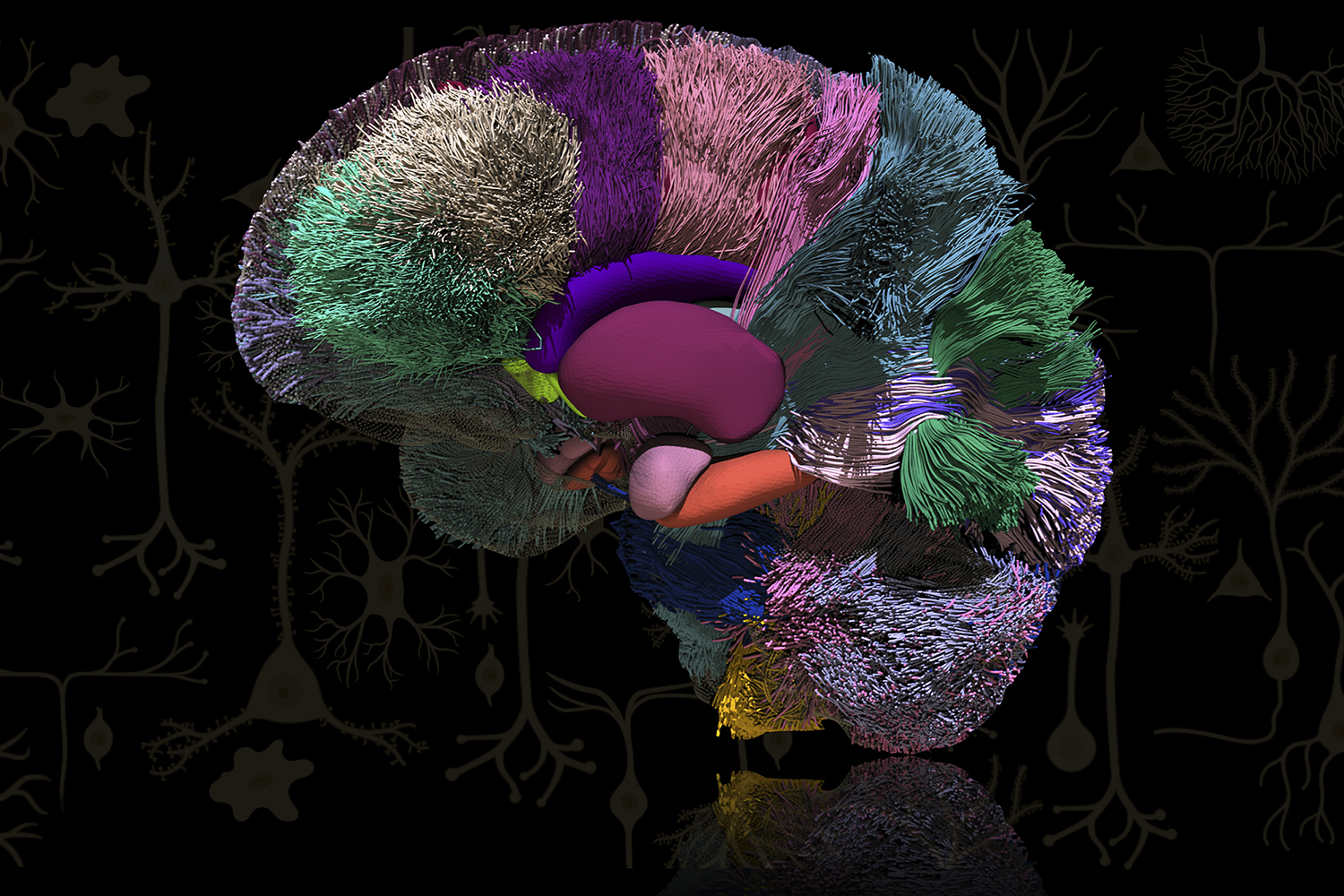
Sahar Ahmad and Ye Wu’s image of the human brain looks drastically different form the one produced by Glass. Both researchers within Pew-Thian Yap’s lab, they wanted to illustrate the enormously complex network of interconnected neurons within the human brain. Using advanced mathematics, they created this highly detailed 3-dimensional image via a medical imaging technique called diffusion magnetic resonance imaging. Research from the Yap Lab targets applications in neuroscience, surgical planning, and radiomics.