When you take a drug to treat serious pain—we’re talking morphine or oxycodone—it alters something called an opioid receptor, part of a certain kind of brain cell. The intended result is relief.
“But there’s a reason why certain medications work well for some people and not others and why those drugs can cause serious side effects, such as seizures, addiction, and death due to overdose,” says Patrick Giguere, a pharmacology postdoc at UNC. “The reason is that we haven’t known the precise biological markers for those drugs.”
Markers represent the biological abnormalities that drugs aim to treat. Currently, many approved drugs—including codeine and Percocet—target opioid receptors, which use a variety of pathways to transmit chemical signals in the brain. “These drugs activate all of the receptor pathways,” Giguere says. “None of them modulate just one pathway.”
This ability of opioids to indiscriminately target receptor pathways is likely responsible for the beneficial and harmful effects associated with these addictive and commonly abused medications.
But now, Giguere and colleagues have found a way to modulate just one pathway. They discovered that tweaking specific amino acids—the building blocks of receptors—drastically changes how opioid receptors control chemical signals.
The finding offers a way to create more precisely targeted therapies, with the potential for enhanced benefit and fewer side effects. And since the opioid system is key for many brain processes, drugs that target these receptors could be useful for many diseases, including depression, chronic pain, and Parkinson’s disease.
At the heart of their discovery is a simple element: salt. A single sodium ion, to be exact.
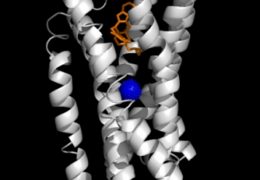
For 40 years, scientists have known that altering sodium concentrations in the brain changed the activity of opioid receptors—the gatekeepers for drugs that treat pain. But no one had been able to figure out precisely how sodium did that. That’s because no one could create a clear picture of what the receptor looked like; researchers couldn’t see what sodium was doing. What they needed was a high-resolution crystal structure of the delta-opioid receptor.
A crystal structure of a tiny piece of brain anatomy is similar to a snow crystal. Both have adopted a solid form that is the most stable form. Water, under specific circumstances, is most stable as a snowflake. A protein, under certain circumstances, is most stable as a crystal.
Yet, snow and protein crystals are formed very differently. A snowflake forms when water freezes onto a dust particle. To create a crystal structure of a protein—such as a brain cell receptor—scientists have to use x-rays and liquid nitrogen to keep the protein stable. For years, this proved difficult in the case of the delta opioid receptor protein because it’s an extremely fragile part of a brain cell’s membrane.
But scientists at the Scripps Research Institute developed a technique that allowed them to create the first ever high-resolution 3-D crystal structure of the delta-opioid receptor. This sharp image revealed a sodium ion at the heart of the receptor.
“Sodium is not everywhere in the receptor,” Giguere says. “It fits in a pocket within the receptor’s structure.”
With that information, Giguere and others in the lab of UNC’s Bryan Roth created experimental procedures to show how specific amino acids hold the sodium ion in place. They also showed how the amino acids and sodium interact in order to modulate brain signals.
“The amino acids control the sodium ion,” Giguere says. “This control is like a trigger; it has a specific function on the opioid receptor.”
When Giguere and UNC colleagues mutated the amino acids, they saw extreme changes in how the delta-opioid receptor responded to chemical signals. In one experiment, Roth’s team tweaked an amino acid to cause a major change in the signaling response of the receptor’s beta-arrestin pathway, which is responsible for shutting down chemical signaling.
These findings suggest that it’s possible to create a drug that targets specific pathways inside the delta-opioid receptor.
Current medications either turn on the opioid receptor or turn it off. Giguere’s work shows how it’s possible to fine-tune the receptor so it functions optimally.
“This is a new field of research, which we call functional selectivity,” Giguere says. “There are very few crystal structures that show how this sort of pathway selectivity can work. This is why we think our findings will lead to another, potentially better class of drugs.”
In other words, when you are in chronic pain, you would get relief without the side effects that give such medications bad reputations.